UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For
the fiscal year ended | |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ______to ______ |
Commission
File Number:
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices, including zip code)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Smaller
reporting company | |
| Emerging
growth company |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No
The
aggregate market value of the voting and non-voting common equity held by non-affiliates on June 30, 2023, the last business
day of the registrant’s most recently completed second fiscal quarter, based upon the closing price of the registrant’s common
stock on such date as reported by The Nasdaq Capital Market, was approximately $
The number of outstanding shares of the registrant’s common stock, $0.0001 par value per share, as of March 25, 2024, was .
DOCUMENTS INCORPORATED BY REFERENCE
RENOVORX, INC. FORM 10-K
TABLE OF CONTENTS
Solely for convenience, trademarks and trade names referred to in this Report may appear without the ® or ™ symbols.
| i |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, or Form 10-K (this “Report”), particularly in the sections captioned “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. All statements other than present and historical facts and conditions contained in this Report, including statements regarding our future results of operations and financial position, business strategy, plans and our objectives for future operations, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “can,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” or “would,” or the negative of these terms or other comparable terminology. Actual events or results may differ from those expressed in these forward-looking statements, and these differences may be material and adverse. Forward-looking statements include, but are not limited to, statements about:
| ● | the sufficiency of our existing cash, cash equivalents, and investments to fund our future operating expenses and capital expenditure requirements; | |
| ● | our estimates regarding expenses, future revenue, anticipated capital requirements to fund our future operating expenses, and our need for additional financing; | |
| ● | our financial performance; | |
| ● | our anticipated use of our existing cash, cash equivalents, and investments; | |
| ● | the ability of our clinical trials to demonstrate safety and efficacy of our product candidates, and other positive results; | |
| ● | the progress and focus of our current and future clinical trials, and the timing of reporting of data from those trials; | |
| ● | our continued reliance on third parties to conduct clinical trials of our product candidates, and for the manufacture of our product candidates; | |
| ● | the beneficial characteristics, safety, efficacy, and therapeutic effects of our product candidates; | |
| ● | our ability to advance product candidates into and successfully complete clinical trials; | |
| ● | our ability to further develop and expand our therapy platform, both to use different chemotherapeutic agents, to include new indications, or to market our catheter on a standalone basis; | |
| ● | enrollment timing and projections for our clinical trials and our expectations relating to the timing of the provision of updates on, data readouts for, and completion of our clinical trials; | |
| ● | our ability to obtain and maintain regulatory approval of our product candidates and the timing or likelihood of regulatory filings and approvals, including our expectation to seek special designations, such as orphan drug designation, for our product candidates for various diseases; | |
| ● | existing regulations and regulatory developments in the United States and other jurisdictions; | |
| ● | our plans relating to commercializing our product candidates, if approved, including the geographic areas of focus and our potential and ability to successfully commercialize our product candidates and generate revenue; | |
| ● | the implementation of our strategic plans for our business and product candidates; | |
| ● | the expected potential benefits of strategic collaborations with third parties and our ability to attract collaborators with relevant and complementary expertise; | |
| ● | our estimates of the number of patients in the United States who suffer from the diseases we target; | |
| ● | our estimates of potential market opportunities and our ability to successfully realize these opportunities; |
| ii |
| ● | the success of competing therapies that are or may become available; | |
| ● | developments relating to our competitors and our industry, including competing product candidates and therapies; | |
| ● | our plans relating to the further development and manufacturing of our product candidates, including for additional indications which we may pursue; | |
| ● | our plans and ability to obtain or protect intellectual property rights, including extensions of existing patent terms where available; | |
| ● | the scope of protection we are able to establish and maintain for intellectual property rights, including our therapy platform and product candidates; | |
| ● | our ability to successfully negotiate and enter into agreements with distribution, strategic and corporate partners; | |
| ● | our potential and ability to successfully manufacture and supply our product candidates for clinical trials and for commercial use, if approved; | |
| ● | our ability to retain the continued service of our key personnel and to identify, hire, and then retain additional qualified personnel; | |
| ● | our ability to maintain compliance with the continuing listing requirements of the Nasdaq Stock Market; and | |
| ● | our expectations regarding the impact of major domestic and geopolitical events on our business. |
We have based the forward-looking statements contained in this Report primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations, prospects, business strategy and financial needs. The outcome of the events described in these forward-looking statements is subject to risks, uncertainties, assumptions and other factors described in the section titled “Risk Factors” and elsewhere in this Report. These risks are not exhaustive. Other sections of this Report include additional factors that could adversely affect our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risks and uncertainties emerge from time to time and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this Report. We cannot assure you that the results, events and circumstances reflected in the forward-looking statements will be achieved or occur, and actual results, events or circumstances could differ materially from those described in the forward-looking statements. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
The forward-looking statements made in this Report relate only to events as of the date on which such statements are made. We undertake no obligation to update any forward-looking statements after the date of this Report or to conform such statements to actual results or revised expectations, except as required by law. Unless the context otherwise indicates, “RenovoRx,” the “Company,” “we,” “our,” and “us” refer to RenovoRx, Inc., a Delaware corporation. All information presented herein is based on our fiscal calendar. Unless otherwise stated, references to particular years, quarters, months or periods refer to the Company’s fiscal years ended in December and the associated quarters, months and periods of those fiscal years.
This Report contains market data and industry forecasts that were obtained from industry publications. These data and forecasts involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such information. We have not independently verified any third-party information. While we believe the market position, market opportunity and market size information included in this Report is generally reliable, such information is inherently imprecise.
| iii |
PART I
ITEM 1. BUSINESS
Overview
We are a clinical-stage biopharmaceutical company developing proprietary targeted combination therapies for high unmet medical needs with a goal to improve therapeutic outcomes for cancer patients undergoing treatment. Our proprietary Trans-Arterial Micro-Perfusion (TAMPTM) therapy platform is designed to ensure precise therapeutic delivery to directly target the tumor while potentially minimizing a therapy’s toxicities versus systemic (intravenous (known as “IV”) therapy).
Our novel and patented approach to targeted treatment offers the potential for increased safety, tolerance, and improved efficacy. Our Phase III lead product candidate, RenovoGemTM, an oncology drug-device combination product, is being investigated under a U.S. Investigational New Drug Application (“IND”) that is regulated by the U.S. Food and Drug Application (“FDA”) 21 CFR 312 pathway. RenovoGem is currently being evaluated for the treatment of locally advanced pancreatic cancer (“LAPC”) by the Center for Drug Evaluation and Research (the drug division of FDA). We also plan to evaluate RenovoGem as a potential therapy in bile duct cancer with other potential pipeline indication opportunities to follow including non-small cell lung cancer, uterine tumors, glioblastoma, and sarcoma.
RenovoGem received FDA Orphan Drug Designation for pancreatic cancer and bile duct cancer, which provides 7 years of market exclusivity upon approval by the FDA of our New Drug Application (“NDA”) for RenovoGem Should such approval be obtained.
We have completed our RR1 Phase I/II and RR2 observational registry studies of RenovoGem, with 20 and 25 patients respectively, in LAPC. These studies demonstrated a median Overall Survival (known as “OS”) of 27.9 months in LAPC patients pre-treated with radiation followed by treatment with RenovoGem. Based on previous large randomized clinical trials, the expected survival of LAPC patients is 12 to 15 months in patients receiving only IV systemic chemotherapy or IV chemotherapy plus radiation (which are both considered standard of care). Unlike the randomized trials that established these standard of care results, our RR1 and RR2 clinical trials did not prospectively control the standard of care therapy received prior to administration of RenovoGem. Based on an FDA safety review of our Phase I/II study, FDA allowed us to proceed to evaluate RenovoGem within our Phase III registrational clinical trial, which is ongoing as described below.
Our Phase III registrational trial of RenovoGem for the treatment of LAPC is called TIGeR-PaC. This clinical trial is an ongoing randomized multi-center study using TAMP to evaluate RenovoGem. The study is evaluating trans-arterial delivery, a form of intra-arterial (“IA”) administration, of an FDA-approved chemotherapy, gemcitabine, to treat LAPC following stereotactic body radiation therapy (“SBRT”). The study is comparing treatment of an FDA-approved cancer drug, gemcitabine, with TAMP versus systemic IV administration of gemcitabine and nab-paclitaxel.
Our protocol for TIGeR-PaC involves systemic chemotherapy and only SBRT during the induction phase of the study (prior to randomization). Patients receiving SBRT during the induction phase are required to complete 5 treatments, over 5 consecutive days, and do not receive oral chemotherapy vs. previously utilized intensity-modulated radiation therapy (IMRT) where patients must complete 25 radiation treatments in combination with oral chemotherapy during the induction phase of the study, which takes between 35 and 56 days to complete. In December 2021, we amended our protocol and statistical analysis plan for TIGeR-PaC (the “Modified SAP”) to (i) analyze only patients receiving SBRT during the induction phase, (ii) include a second interim analysis, (iii) change the total number of patients randomized in the study to 114 with a total of 86 deaths from SBRT patients, and (iv) repower the study from 90% to 80%, which is commonly used in clinical trials. We believe this design will shorten the timeframe needed to complete the study and also significantly decrease our costs. We have not discussed the protocol amendment or the Modified SAP with the FDA, and we cannot provide any assurance that the FDA will agree with these modifications, but these modifications have been submitted to the FDA.
| 1 |
The first interim analysis in the TIGeR-PaC study at the 26th event of the specified events (deaths), was completed in March 2023, with the Data Monitoring Committee recommending a continuation of the study. The TIGeR-PaC study’s primary endpoint is a 6-month OS benefit with secondary endpoints including reduced side effects versus standard of care. The data was first presented at the 2023 American Association for Cancer Research Annual Meeting in April 2023 and then as a Late Breaker Oral Presentation with additional secondary endpoint data at the 2023 European Society of Medical Oncology World Congress on Gastrointestinal Cancer in June 2023. The second interim analysis for this study will be triggered by the 52nd event (death of patient), which is estimated to occur in late 2024. The second interim data read out would follow thereafter, with the timing for such read out depending on customary factors such as time needed for analysis.
Our TAMP therapy platform is focused on optimizing drug concentration in solid tumors using approved small molecule chemotherapeutics. Our platform enables physicians to isolate segments of the vascular anatomy closest to tumors and force chemotherapy across the blood vessel wall to bathe these difficult-to-reach solid tumors in chemotherapy. Specifically, our patented approach allows physicians to combine, on the one hand, pre-treatment of the local blood vessels and tissue with standard-of-care radiation therapy to decrease chemotherapy washout and, on the other hand, local delivery via our patented RenovoCath delivery system which utilizes pressure to force small molecule chemotherapy into the tumor tissue. We believe there are many advantages to our TAMP therapy platform, including:
| ● | Application of Approved Small Molecule Chemotherapeutic Agents: To date have used approved small molecule chemotherapeutic agents, such as gemcitabine, with well-known safety and efficacy profiles. | |
| ● | Targeted Approach: In a preclinical study using our therapy platform, we demonstrated up to 100 times higher local drug concentration compared to systemic chemotherapy. We believe our TAMP therapy platform allows for a targeted approach that can decrease systemic exposure and improve patient outcomes. | |
| ● | Delivery Method Independent of Tumor Vascularity: Our therapy platform is designed to deliver therapy to solid tumors resistant to systemic therapy due to lack of tumor feeder blood vessels. If approved, our product candidates have the potential to treat tumors that are not directly supported by blood vessels. | |
| ● | Broad Application for Solid Tumor Indications: Our therapy platform is not restricted to a single small molecule chemotherapeutic agent or solid tumor type. As such, it may be utilized for delivery of other agents and applied for use in additional solid tumor indications, including in solid tumors without identifiable tumor feeder blood vessels. |
| 2 |
In further validation of our TAMP platform, in July 2023, we announced a collaboration with Imugene (ASX: IMU) to explore expansion of our TAMP product pipeline with Imugene’s CF33 oncolytic virus therapy for the treatment of difficult-to-access tumors. We are constantly in discussions regarding similar collaborations and potentially out-licenses of RenovoGem as we gear up for the RenovoGem NDA filing (assuming we meet our study endpoints) and commercialization of RenovoGem (if approved by FDA) as well as other potential collaborations with our TAMP platform.

Figure 1: Graph showing first interim analysis in the TIGeR-PaC study
Research and Development Pipeline
Our portfolio of cancer therapies is based on our lead product candidate, RenovoGem (gemcitabine delivered via our patented delivery system), regulated by the FDA as a novel oncology drug product. RenovoGem utilizes pressure-mediated delivery of gemcitabine across the arterial wall to bathe tumor tissue in chemotherapy.
Funding permitting, we plan on further evaluating RenovoGem in other cancer indications. Our current pipeline is summarized below:
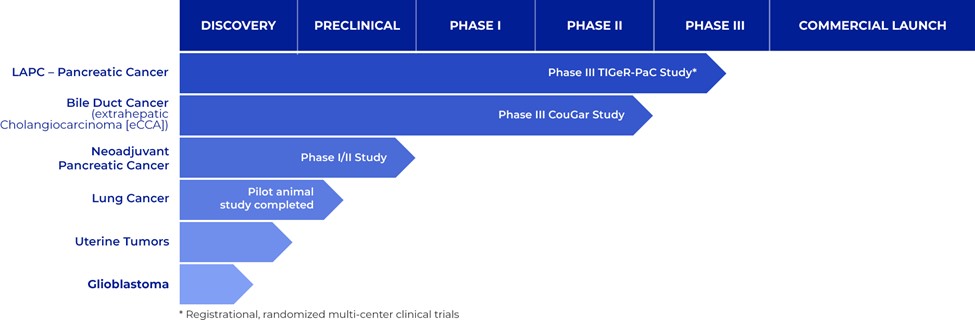
Figure 2: RenovoGem Clinical Pipeline detailing our potential portfolio of cancer therapies based on our TAMP therapy platform.
Gemcitabine has been considered a standard of care drug for several solid tumors, and the drug’s anti-cancer tumor effects are well profiled. Our TAMP platform therapy utilizes pressure mediated delivery of gemcitabine across the arterial wall to bathe the pancreatic tumor tissue in 120 mL of saline with 1,000 mg/m2 of the drug over a 20-minute delivery period (delivering 1,500-2,000 mg of drug depending upon patient body surface area). Our delivery system, RenovoCath, is a double balloon catheter designed with the capability to isolate sections of the blood vessel through the adjustment of the distance between the balloons, thereby excluding any branching blood vessel offshoots in order to create the pressure head needed to push drug across the blood vessel wall.
| 3 |
We intend to explore applications of our TAMP platform in additional indications, including bile duct cancer, locally advanced lung cancer, locally advanced uterine cancer, and glioblastoma. We have completed and presented data on a lung cancer application in preclinical studies, and additional preclinical experiments in lung cancer may be conducted.
We are using gemcitabine in our initial anti-cancer product candidate, RenovoGem. However, multiple small molecule therapeutics are compatible with our TAMP platform. We intend to opportunistically develop additional anti-cancer product candidates using small molecule therapeutics in combination with our therapy platform.
While the field of oncology has seen progress in treating a handful of deadly cancers over the last few decades, there is a common objective in chemotherapy: enhanced dosing of the drug to impact the tumor while minimizing systemic toxicity. The characteristics of the blood vessels, within and surrounding the tumor, can limit or thwart the achievement of this goal. For example, LAPC and bile duct cancer, known as extrahepatic (or outside the liver) cholangiocarcinoma (“eCCA”) are more difficult to treat due to the lack of blood vessels that feed these tumors, making it difficult to expose tumors to chemotherapy, which is typically delivered intravenously. Trans-arterial chemoembolization (“TACE”) is an established first line therapy for solid tumors. A key component of this approach is to identify and isolate vessels feeding the tumor, known as tumor feeder blood vessels. However, in patients with pancreatic cancer, no tumor feeder blood vessels are visible despite attempts to image them using a variety of modalities. In the absence of visible tumor feeder blood vessels, our therapy platform has the potential to introduce drugs directly across the arterial wall into the surrounding tissue via pressurized diffusion.
RenovoGem in Locally Advanced Pancreatic Cancer (LAPC)
Our protocol for the TIGeR-PaC clinical trial involves systemic chemotherapy and only SBRT during the induction phase of the study (prior to randomization). Patients receiving SBRT during the induction phase are required to complete 5 treatments, over 5 consecutive days, and do not receive oral chemotherapy vs. previously utilized intensity-modulated radiation therapy (“IMRT”) where patients must complete 25 radiation treatments in combination with oral chemotherapy during the induction phase of the study, which takes between 35 and 56 days to complete. In December 2021, we amended the protocol as follows: we plan to (i) analyze only patients receiving SBRT during the induction phase, (ii) include a second interim analysis, (iii) change the total number of patients randomized in the study to 114 with a total of 86 deaths from SBRT patients, and (iv) repower the study from 90% to 80%, which is commonly used in clinical trials. We believe this design will shorten the timeframe needed to complete the study and also significantly decrease our costs. We have not discussed the protocol amendment or the Modified SAP with the FDA, and we cannot provide any assurance that the FDA will agree with these modifications, but these modifications have been submitted to the FDA.
The first interim analysis in the TIGeR-PaC study at the 26th event of the specified events (deaths), was completed in March 2023, with the Data Monitoring Committee recommending a continuation of the study. The TIGeR-PaC study’s primary endpoint is a 6-month OS benefit with secondary endpoints including reduced side effects versus standard of care. The data was first presented at the 2023 American Association for Cancer Research Annual Meeting in April 2023 and then as a Late Breaker Oral Presentation with additional secondary endpoint data at the 2023 European Society of Medical Oncology World Congress on Gastrointestinal Cancer in June 2023. The second interim analysis for this study is expected to occur at the 52nd event which is estimated to occur in late 2024.
RenovoGem in eCCA and Other Potential Clinical Indications
Funding permitting, we also plan to evaluate RenovoGem as a potential therapy in bile duct cancer with other potential pipeline indication opportunities to follow including non-small cell lung cancer, uterine tumors, glioblastoma, and sarcoma.
After significant input from key opinion leaders across the spectrum of relevant medical specialties and feedback from the FDA, we submitted the protocol for a Phase II/III eCCA clinical trial to FDA, and after receiving feedback, we are finalizing the protocol and also including incorporating a recently approved drug, durvalumab into the study protocol with guidance from the Steering Committee. We anticipate launching this study mid-2024. We have also secured FDA Orphan Drug Designation for RenovoGem for the treatment of cholangiocarcinoma, which would provide us with seven years of orphan exclusivity to market RenovoGem for our eCCA indication upon NDA approval, provided that we are the first sponsor to obtain FDA approval for IA gemcitabine for the eCCA indication.
| 4 |
Our Team
Our management team, Board of Directors, and Scientific Advisors provide us with expertise across multiple sectors to drive success through clinical development and subsequent commercialization of our novel therapy platform. Our Chief Executive Officer, Shaun Bagai, gained extensive experience running clinical trials and launching, creating, and developing new markets for novel therapies at TransVascular, Medtronic, Ardian, and HeartFlow. Dr. Ramtin Agah, our Co-Founder and Chief Medical Officer, is a practicing cardiovascular specialist who has 20 years of research experience in vascular biology and disease in both academia and industry. Leesa Gentry, our Chief Clinical Officer, with 29 years of experience focused on improving clinical research programs in Contract Research Organization (CRO), pharmaceutical, and biotech industries. Past employers include IQVIA (Quintiles), PPD, OmnicareCR and Otsuka. Prior to RenovoRx, she was Senior Vice President (SVP) of Clinical Operations at Evotec. Our Board of Directors includes a wide range of public and private company management, board and life sciences experience, including drug/device combination and oncology experience. Clinical advisors include experts across many specialties who treat solid tumors. Dr. Margaret Tempero is Professor of Medicine, Division of Hematology and Oncology, at UCSF. She also serves as Chair of the NCCN Guidelines Panel on Pancreatic Cancer (since 2000), editor-in-chief of JNCCN, and on the ASCO Conquer Cancer Foundation Board. Previously, Dr. Tempero has served on the ASCO Board of Directors, as ASCO President, and as a member of FDA’s Oncology Drug Advisory Committee. Dr. Michel Ducreux is the Head of the Gastrointestinal Oncology Unit and Gastrointestinal Oncology Tumor Board at Gustave Roussy, Professor of Oncology at Paris-Saclay University in France, and Vice-Chair of ESMO GI. Dr. Michael Pishvaian, a medical oncologist, has extensive experience running oncology studies and is an Associate Professor, and Director of the Gastrointestinal, Developmental Therapeutics, and Clinical Research Programs at the NCR Kimmel Cancer Center at Sibley Memorial Hospital Johns Hopkins University School of Medicine. Dr. Pishvaian is the Principal Investigator / Global Study Chair of our TIGeR-PaC Phase III study. Dr. Karyn Goodman serves as the Radiation Monitor for our TIGeR-PaC Phase III study and Professor and Vice Chair of Clinical Research, Department of Radiation Oncology at the Icahn School of Medicine at Mount Sinai, and Associate Director of Clinical Research at the Tisch Cancer Institute at Mount Sinai.
Current Treatments and Limitations of Approaches
Currently, solid tumors are typically treated using one or a combination of treatment modalities: surgery, radiation, and pharmacological therapies (chemotherapy). For solid tumors, when possible, surgical resection of the tumor is the most frequently employed treatment approach. If the tumor is detected at an early stage and is localized to the affected organ, surgical removal of the entire tumor may be an effective and potentially curative treatment. In most cases, surgery is undertaken and / or completed prior to commencing additional treatment approaches. However, multiple solid tumor types, including LAPC and eCCA are diagnosed at advanced stages, which precludes surgery as a treatment approach. In many of these circumstances, the tumor has grown into adjacent anatomical structures making surgery difficult or impossible.
IV, also known as systemic, chemotherapy (gemcitabine and nab-paclitaxel), which has a seven-week survival benefit over IV gemcitabine alone, is considered standard of care for most solid tumors, but limitations include less than acceptable efficacy, systemic toxicities, and other side effects.
For the treatment of some localized solid tumors, TACE is an established first line therapy. Many companies have developed therapeutic products for use in this approach to treat tumors of the liver, uterus, and prostate. Many solid tumors have a dedicated blood supply: small blood vessels, called tumor feeder blood vessels, that branch off of larger native arteries and terminate in the tumors to provide nutrition to the tumors. A key aspect of TACE is to identify and isolate these tumor feeder blood vessels during x-ray angiography and then deliver the desired therapy including chemotherapy and embolic agents. In patients with LAPC, no tumor feeder blood vessels are visible during angiography due to the avascular (lack of blood vessels) nature of these tumors. This limitation has rendered TACE ineffective in the treatment of patients with LAPC, eCCA, and a subset of other solid tumors. The limitations of TACE translate to low survival rates in these tumor subtypes. The use of TACE with or without immuno-oncology treatment approaches, which harness the body’s immune system to treat cancer, has not significantly improved survival rates in these subtypes. For example, due to the inability of immune cells to penetrate the tumor tissue, early studies of targeted immunotherapies in pancreatic cancer have demonstrated limited success.
| 5 |
Our Platform: TAMP
TAMP may work best with avascular tumors
Certain tumor types are sufficiently vascularized (i.e., tumors with blood vessels associated with them) to enable use of systemic chemotherapy and standard of care local therapy techniques. In Figure 3 below, for example, the panel on the left depicts visualization of an actual tumor, hepatocellular carcinoma (“HCC”), or primary liver cancer, under x-ray angiography as dye injected through the arteries reaches the tumor itself. Further, visible tumor feeder blood vessels can be reached by simple end-hole catheters to deliver targeted therapy to these liver tumors. In contrast, the panel on the right illustrates the typical lack of tumor feeder blood vessels to a pancreatic tumor. Given the lack of tumor feeder blood vessels, the dye does not reach the tumor, rendering the tumor “invisible” under x-ray angiography.
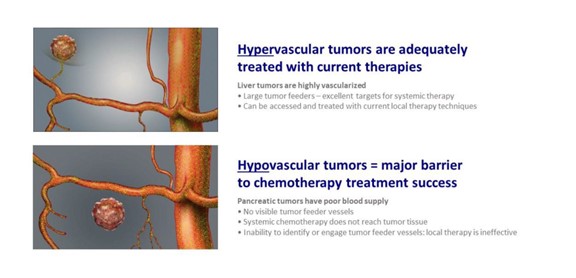
Figure 3: Showing liver tumors that are highly vascularized, and pancreatic tumors that are avascular.
TAMP has been under development for over 14 years
In 2009, our founder Dr. Ramtin Agah, an experienced interventional cardiologist with a degree in biomedical engineering, developed the concept for TAMP as a way to deliver chemotherapy locally to treat poorly vascularized tumors. He joined forces with Kamran Najmabadi, who brought significant medical device engineering experience, to found RenovoRx in 2009. Subsequently, we engaged a contract manufacturer to prototype and manufacture our RenovoCath delivery devices. We received our first FDA 510(k) clearance for RenovoCath in 2014, a second clearance to use the RenovoCath for infusion of chemotherapy agents in 2017, a further clearance to use RenovoCath with a power-injector in 2019, and a fourth clearance in 2021 to expand vessel diameter range to 3-11mm, implement certain changes in the Instructions for Use, change the recommended saline to contrast solution ratio, among other changes and improvements. RenovoCath is intended for the isolation of blood flow and delivery of fluids, including diagnostic and/or therapeutic agents, to selected sites in the peripheral vascular system. RenovoCath is also indicated for temporary vessel occlusion in applications including arteriography, preoperative occlusion, and chemotherapeutic drug infusion. RenovoCath is intended for general intravascular use in the peripheral vasculature in arteries 3 mm and larger as well as for use in arteries from 3 mm in diameter for vessel entry and to occlude vessels ranging between 3 mm to 11 mm in diameter. We are evaluating our lead product candidate RenovoGem under an IND filed in 2018. FDA has determined that RenovoGem will be regulated as, and if approved we expect will be reimbursed as, a new oncology drug product.
| 6 |
How it works: we developed TAMP as an attempt to solve the problems of treating avascular tumors
To overcome the limitations resulting from a lack of tumor feeder blood vessels, we explored a different approach to locally deliver anti-cancer drugs. By isolating a section of the blood vessel and then increasing the intravascular pressure in the isolated segment, we can introduce chemotherapy directly across the arterial wall into the surrounding tissue via pressurized diffusion, which we call Trans-Arterial Micro-Perfusion (for the acronym TAMP). To isolate the vessel and create this pressure gradient, we developed RenovoCath, a patented adjustable double balloon catheter to occlude the proximal and distal part of the vessel. Using the TAMP technique in explanted (dissected out of the animal and used separately in a saline water bath) pig aorta and iliac arteries (peripheral arteries that carry blood to the legs, reproductive organs and pelvis), we were able to validate our hypothesis by demonstrating >99% gemcitabine pressurized diffusion across the arterial wall in the absence of feeder vessels. This mechanism of action was further supported by exploratory acute animal studies measuring the pressure gradient within the artery during double balloon occlusion. Figure 4 demonstrates the change in IA pressure over time from catheter introduction to balloon inflation, start of infusion, and pressure plateau when chemotherapy is forced out of vessel. These changes in pressure are a result of pressure declining as the first balloon blocks blood inflow and then rising as the drug is administered and fills up the space between the balloons.
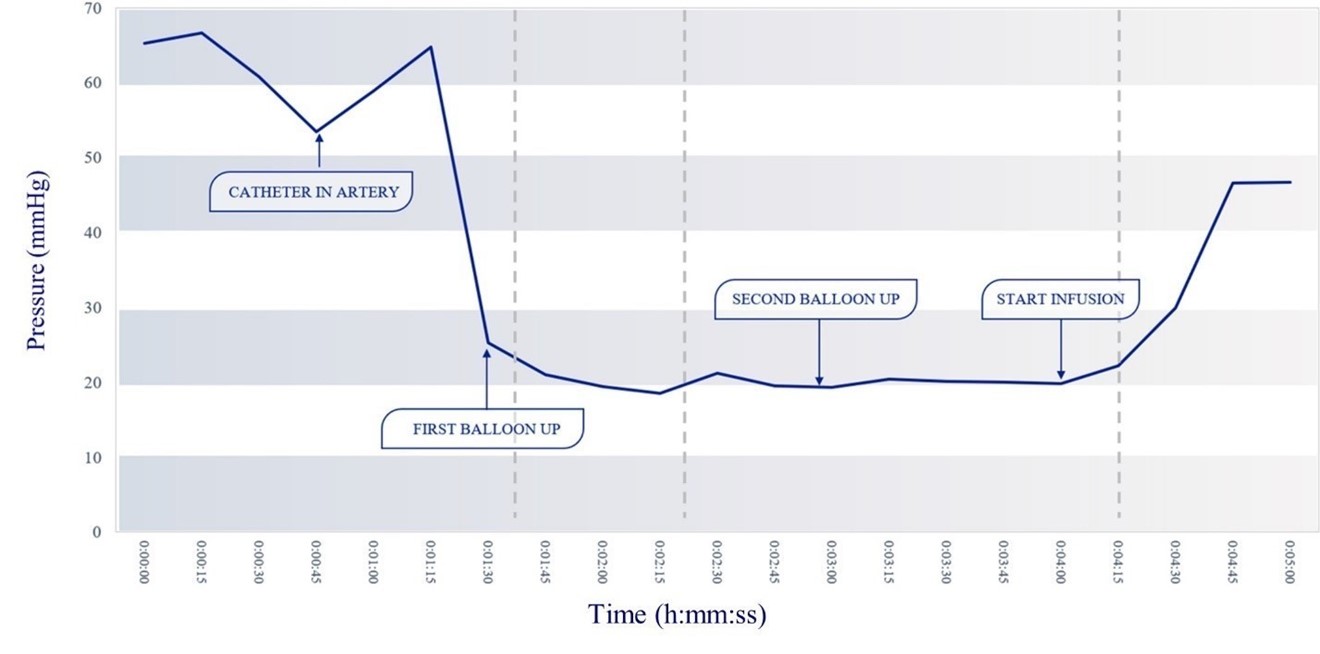
Figure 4: Occluding the vessel with RenovoCath, while adjusting the balloon-to-balloon distance to exclude all blood vessel branches, established an intravascular interstitial pressure in the isolated blood vessel segment of approximately 20 mmHg. With subsequent infusion of fluids between the balloons at 6 mls/minute, the intravascular pressure increases to above 45 mmHg, trans-arterially forcing the small molecule drug across the arterial wall via diffusion (this patented process of perfusing the vessel wall is Trans Arterial Micro Perfusion or TAMP).
Our TAMP platform therapy utilizes pressure mediated delivery of gemcitabine across the arterial wall to bathe the pancreatic tumor tissue in 120 mL of saline with 1,000 mg/m2 of drug over a 20-minute delivery period (delivering 1,500 - 2,000 mg of drug depending upon patient body surface area. This blanketing approach of large fluid volume delivery over time may enable the drug to approach these difficult-to-reach tumors.
We believe some of the key advantages of TAMP include:
| ● | It is ideal for solid tumors where resection is not possible due to proximity/impingement of tumor on blood vessels, nerves, or other key structures | |
| ● | No need for identifying tumor feeder blood vessels to deliver the drug. These generally do not exist in avascular or hypovascular tumors such as LAPC and eCCA | |
| ● | In solid tumors without identifiable feeder vessels, TAMP is technically easier than direct cannulation of small tumor feeder blood vessels | |
| ● | High local concentration of drug into the tumor tissue | |
| ● | Potential for decreased systemic exposure of drug due to local metabolism prior to systemic exposure |
| 7 |
Developing a therapeutic platform using an adjustable two-balloon catheter and IA gemcitabine
By isolating the vessel adjacent to the tumor and creating a pressure gradient across the arterial wall between the isolated vessel segment and the surrounding tissue or tumor, we are able to force the small molecule chemotherapy across the vessel directly into surrounding tissue or tumor. To accomplish this, we needed a minimally invasive technique to isolate the blood vessel next to the tumor, exclude any branches that can cause washout of chemotherapy away from the target, and then infuse the chemotherapy into the isolated segment to achieve pressure mediated diffusion through the vessel wall and into the tumor tissue. We accomplished this with our patented RenovoCath delivery system. RenovoCath is a double balloon catheter designed with the capability to isolate the proximal and distal sections of the vessel through the adjustment of the distance between the balloons, thereby excluding any branching blood vessel offshoots. Using standard interventional techniques, an interventional radiologist inserts the RenovoCath delivery system into the body through the femoral artery and positions it in the artery closest to the tumor. Once the balloons are inflated and the position is confirmed, chemotherapy is delivered through the handle, exiting the device between the balloons. It is forced through the vessel wall into the tissue over a 20-minute period. The RenovoCath delivery system is depicted below in Figure 5.
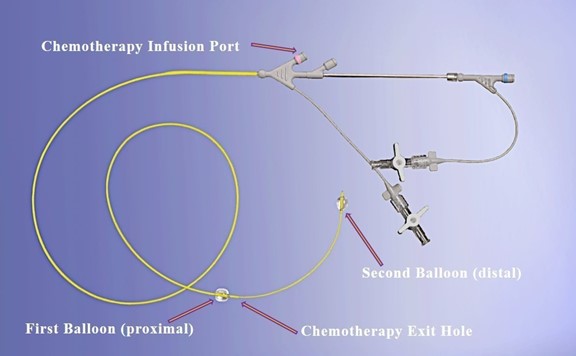
Figure 5: RenovoCath delivery system illustrating two balloon configuration to isolate the target vessel segment, and chemotherapy delivery port and exit hole.
After the procedure is complete, RenovoCath is discarded, and the patient is generally discharged the same day. On average, the entire procedure takes approximately 90 minutes. According to the TIGeR-PaC study protocol, IA treatment is administered through RenovoCath every other week for a maximum of 8 treatments for approximately 16 weeks. Interventional radiologists using the device are typically proctored for their first 2-3 cases only. In addition, platform training for our primary indication should transfer to other indications.
| 8 |
RenovoGem for LAPC
Disease Overview
Pancreatic cancer is one of the deadliest cancers in the U.S. with very poor outcomes. According to American Cancer Society’s Cancer Facts & Figures 2023, pancreatic cancer has a 5-year combined overall survival rate of 12% (Stages I-IV) and is on track to be the second leading cause of cancer-related deaths before 2030. LAPC is diagnosed when the disease has not spread far beyond pancreas, however, has advanced to the point where it cannot be surgically removed. LAPC is typically associated with patients in stage 3 of the disease as determined by the TNM (tumor, nodes and metastasis) grading system.
Current Treatment Landscape and Limitations
Pancreatic cancer has limited treatment options including one or a combination of surgery, radiation, chemotherapy, and/or some targeted therapies. Only a small subset of pancreatic cancer patients is eligible for surgery (“Resectable” at the time of presentation (Stage I-II: 15%); the rest are distributed between having tumors with unresectable LAPC (Stage III: 30%) and metastatic pancreatic cancer (Stage IV: 50%).
Chemotherapy is at the forefront of systemic therapy for cancer. It can be used in the neoadjuvant (before surgery) setting to attempt to decrease tumor size in resectable or borderline resectable patients, in the adjuvant (after surgery) setting, or first line in the metastatic/advanced setting. The backbone of our first product candidate, gemcitabine, is a nucleoside metabolic inhibitor that exhibits antitumor activity by blocking the synthesis of new DNA, which results in cell death. Gemcitabine administered as an IV infusion has an established role in the treatment of both unresectable LAPC and metastatic pancreatic cancer. Since its introduction in the US as Gemzar® (gemcitabine for injection) in 1996 with an FDA approved indication as such, it remains in the guidelines as standard of care. It has been demonstrated to provide clinical benefit for subjects (decreased pain and improved performance status) as well as to improve the time to tumor progression and survival for subjects with metastatic pancreatic cancer and LAPC. However, major improvement in the survival curve of all pancreatic cancer subjects has been a clinical challenge, with an average median survival time for LAPC stalled at 12-15 months from time of diagnosis.
A key limitation of conventional chemotherapy in these tumors can be attributed to their avascular nature and desmoplasia (fibrosis or the growth of scar tissue) that impedes drug delivery. Pancreatic tumor cells have a thick and poorly perfused stroma, or connective tissue, and high interstitial pressure. This can potentially constrict blood vessels leading to an avascular or hypovascular environment that impedes chemotherapy from reaching tumor cells in high enough volume, rendering them relatively resistant to chemotherapy.
In patients with metastatic disease, two chemotherapy combination regimens have shown superiority to gemcitabine, albeit with increased toxicity. First, the combination of oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) in a relatively young cohort of metastatic pancreatic cancer patients appears superior to gemcitabine by improving survival from 6.8 to 11.1 months. Second, in the Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) trial, the combination of gemcitabine plus nab-paclitaxel (Abraxane) demonstrated an OS benefit of 9 weeks versus gemcitabine alone at the cost of increased toxicity.
A major focus of clinicians is determining the optimal method to treat patients with LAPC, patients with localized disease who are not surgical candidates, roughly 30% of all pancreatic cancer patients. IV, or systemic, administration of chemotherapy has yielded unsatisfactory results in these patients. Various localized treatments have included high dose local radiation, direct attempts at local injection of drugs, and use of adenoviral vectors to deliver toxic agents. These treatment options demonstrated limited success in the treatment of LAPC. The lack of successful treatment options represents a recognized unmet medical need for these patients.
Standard of care chemotherapy for the treatment of pancreatic cancer has historically shifted a couple of times with the addition of erlotinib to gemcitabine 15 years ago resulting in a 14-day survival benefit. In 2013, the addition of Abraxane to gemcitabine was approved, with immediate deep market penetration based on an 8-week survival benefit despite higher systemic drug toxicities.
| 9 |
Our Solution
We believe that our product candidate, RenovoGem, has the potential to address the recognized unmet medical need. Utilizing our patented TAMP therapy platform, we believe RenovoGem can enhance local drug concentration, thereby increasing efficacy and decreasing systemic exposure and toxicity to improve patient outcomes. RenovoGem is a drug/device combination product candidate consisting of IA gemcitabine and our proprietary RenovoCath delivery system which forces the anti-cancer drug into the tumor. RenovoGem is regulated by the FDA as a new oncology drug product. While we may explore commercial opportunities to sell RenovoCath as a standalone catheter, our main focus currently is to sell RenovoCath in combination with IA gemcitabine (as RenovoGem) or potentially with other therapeutic agents.
Based on primary market research and analysis of the U.S. market sponsored by us and conducted by third parties, we believe that over 5,000 patients per year would be excellent candidates and undergo RenovoGem treatment if and once it is approved in the U.S. The independent oncologists interviewed stated their dissatisfaction with current standard of care and the strong desire for a therapy like ours to extend potential survival while maintaining quality of life. Further, the analysis suggests, based on analogous oncology drugs with only a modest efficacy benefit, a novel drug can expect 50-80%+ penetration in a first line setting. The results of the Key Opinion Leader, or KOL interviews revealed that a majority of oncologists would refer 90%+ of their LAPC patients who are eligible for the procedure for TAMP if the current Phase III trial demonstrates at least a 4-month survival benefit over systemic chemotherapy. As of March 8, 2023, we announced interim analysis results of the study suggesting a 6-month potential improvement in median overall survival with RenovoGem, pending ongoing clinical investigation. We believe this first-of-two interim analyses indicates that the TIGeR-PaC study is on track to demonstrate increased lifespan for patients being treated with RenovoGem for LAPC.

Figure 6: We invented a new therapy platform, TAMP, that uses pressure to force small molecule chemotherapeutics across the vessel wall into the surrounding tissue using our patented RenovoCath delivery system. Our first product candidate, RenovoGem, is a drug/device combination of IA gemcitabine and the RenovoCath delivery system, and is under development for LAPC and eCCA. We have secured Orphan Drug Designations for RenovoGem for the treatment of both pancreatic cancer and cholangiocarcinoma.
| 10 |
Clinical Development of RenovoGem in LAPC
Preclinical Studies and Data
Once RenovoCath is introduced via standard interventional technique to the arterial vessel segment next to the targeted tissue, both balloons are inflated, and the vessel segment is isolated from the rest of the circulatory system. With inflation of balloons, the pressure is observed to drop within the vessel. However, with infusion of fluids between the balloons, the intravascular pressure increases beyond 45 mmHg until plateauing, generating a gradient and trans-arterially forcing the infusate across the arterial wall via diffusion or Trans-Arterial Micro-Perfusion (i.e., TAMP). A key aspect of this approach is to adjust the distance between the balloons to exclude any side blood vessel branches in the isolated segment to allow the increase in pressure gradient, rather than drug washout via the side branches. Figure 7 shows a comparison, in an animal study, between proper balloon positioning with no side branches, allowing maximum drug to cross the arterial wall, versus improper balloon positioning to include side branches, resulting in drug washing out via the side branches.
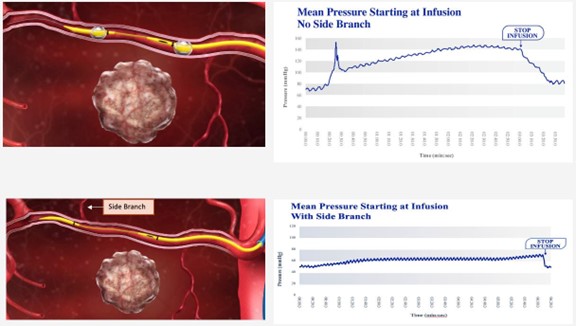
Figure 7: Top panel demonstrates proper balloon positioning with no side branch. Pressure increases with infusion and reaches a plateau of approximately 75 mmHg higher than initial pressure. Bottom panel demonstrates improper balloon positioning with side branch between the balloons. Pressure increases with infusion and reaches a plateau of approximately only 15mmHg higher than initial pressure.
| 11 |
With diffusion of fluids across the arterial wall in TAMP, we expected to be able to deliver small molecules into the surrounding tissue. We performed the following studies to validate this hypothesis:
| 1) | In a preclinical study, 99% of the gemcitabine crossed the arterial wall via TAMP. |
In explanted (dissected out of the animal and used separately in a saline water bath) pig iliac and aortic artery, with the introduction of RenovoCath and infusion of gemcitabine in the isolated vessel segment, we were able to measure (in a time dependent fashion) the amount of gemcitabine crossing the arterial wall into the surrounding fluid. We isolated the arterial vessel segment using RenovoCath and then delivered 60 mg/minute of gemcitabine into the isolated area over 20 minutes. By the end of the infusion, we measured 1,188 mg of gemcitabine in the surrounding fluid around the vessel and 9 mg in the analyzed tissue of the vessel. This demonstrated that 99% of the drug crosses the arterial wall and only 0.75% is retained in the arterial tissue (Figure 8).
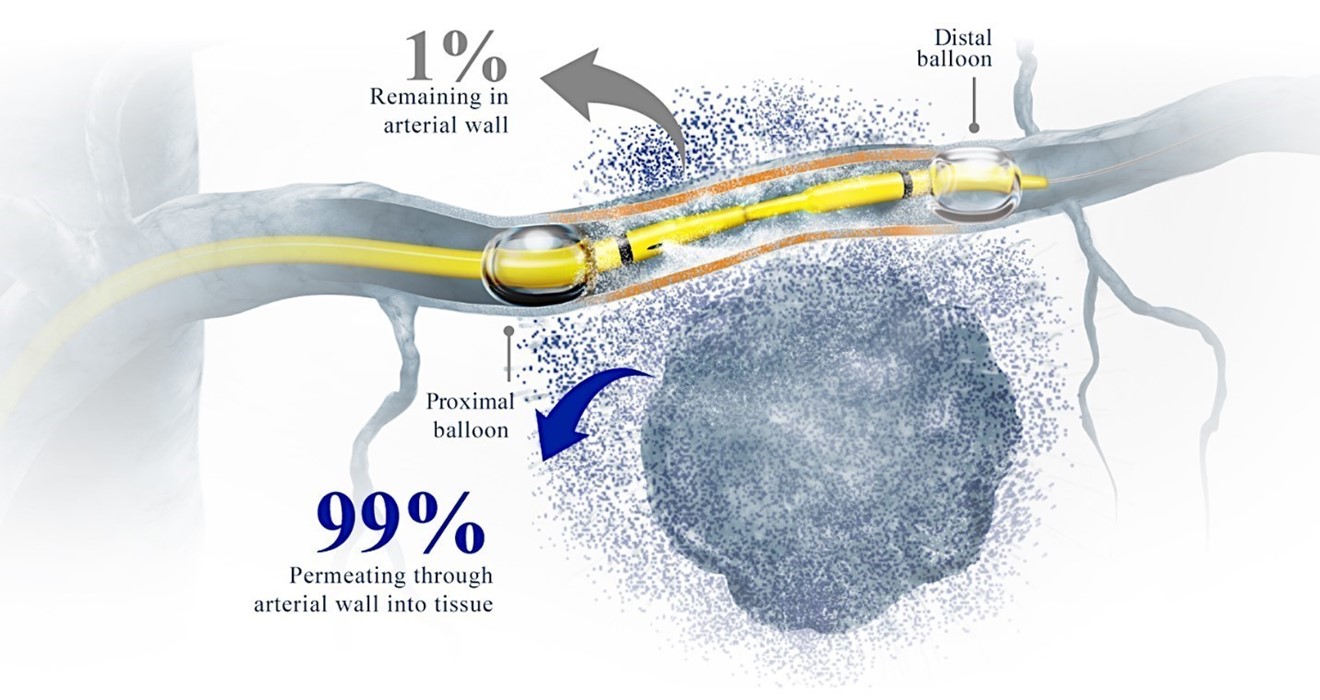
Figure 8: TAMP: delivery of chemotherapy through the RenovoCath and into the tissue to bathe the tumor in chemotherapy. In a preclinical study using gemcitabine, 99% of the drug crosses the arterial wall and less than 0.75% is retained in the vessel wall tissue.
| 2) | Infusion of gemcitabine via TAMP has demonstrated vascular safety with acceptable toxicity in a pig model and does not cause loss of vessel integrity or inflammation. |
Six pigs were treated with gemcitabine via TAMP (6 mL/min for 20 minutes). Target vessels included selection of the superficial femoral artery (SFA) and splenic arteries from each animal (either test or saline control). A total of 6 vessels (3 SFA and 3 splenic arteries) were treated with an equal number of control vessels. All animals survived the 7-day in-life period although two of the animals with gemcitabine treatment in the splenic artery experienced atypical pain during the post-operative phase and required additional pain management with eventual complete recovery.
Analysis of the vessels demonstrated preserved vessel shape with intact endothelial cells (cells on the inside of the vessels). Minimal to no inflammation was observed. The only vessel toxicity observed was a reduction of smooth muscles cells in the vessel wall, primarily close to the inside of the vessel.
| 3) | In preclinical studies, TAMP achieved targeted local drug (dye) delivery. |
I. Targeted small molecule delivery (dye) into pancreatic tissue
We further validated our approach for tissue drug delivery using acute animal experiments. Using both dye and gemcitabine infusion via the TAMP therapy, we were able to demonstrate that fully isolating a segment of a vessel (by blocking inflow and outflow in the target vessel as well as side branches with the RenovoCath double balloons) can lead to dye penetration greater than 4.0 cm from the vessel wall and drug tissue concentration (gemcitabine) up to 100-fold greater than systemic administration.
| 12 |
In an acute pig experiment, RenovoCath was introduced into the gastro-duodenal artery (GDA), a side branch was excluded (using small implants that block the artery, coils), and then dye was introduced at 6mls/minute over 2 minutes. Analysis demonstrated that the blue dye diffused covered approximately 10.56 cm2 (2.2 cm x 4.8 cm) of the pancreas.
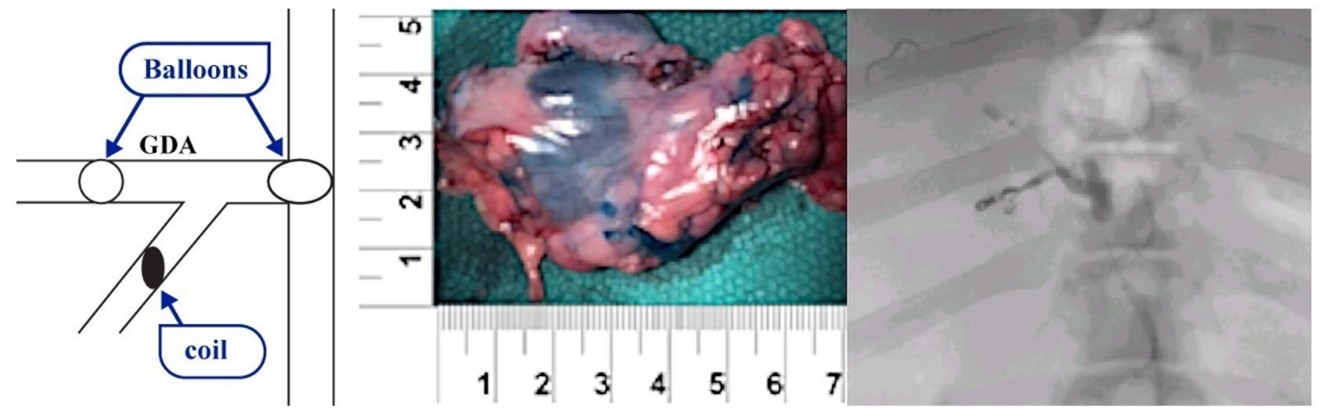
Figure 9: RenovoCath was introduced into the GDA and a side branch was excluded by coiling. This test was conducted in an acute porcine model and demonstrated a dye coverage area of approximately 10.56 cm2 for a 2-minute dye infusion. All dimensions in above figure are in cm.
The study was repeated in 6 other vessel targets to validate the impact of vessel isolation on dye penetration into the surrounding tissue with similar results.
II. Small molecule delivery (dye and gemcitabine) locally into lung tissue
In another set of acute animal experiments, the pulmonary artery was isolated via access through the internal jugular vein. Six ml of methylene blue dye was injected over 1 min and gemcitabine was subsequently delivered locally at rate of 6 mls/minute for 20 minutes to the lung tissue using the TAMP procedure.
| 13 |
Dense dye staining localized to the area of the isolated vessel segment was observed. Again, analysis established penetration into surrounding tissue (4 cm). Furthermore, TAMP achieved greater than 100-fold tissue concentration of gemcitabine versus the tissue level achieved by IV (systemic) delivery of gemcitabine at the same infusion rate.
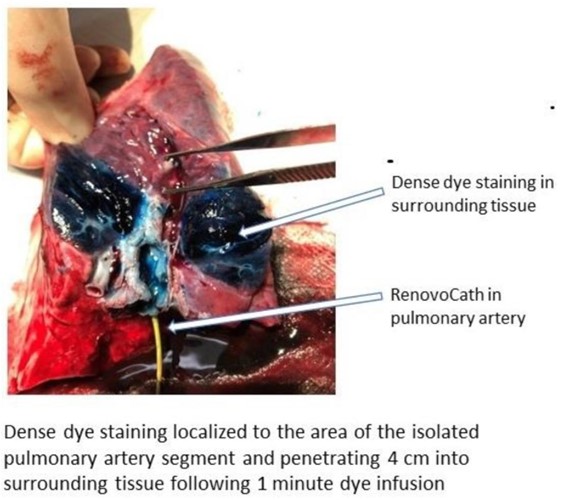
Figure 10: Dense dye staining localized to the area of the isolated pulmonary artery segment and penetrating 4 cm into surrounding tissue following 1 minute dye infusion. In addition, gemcitabine was delivered via TAMP for 20 minutes demonstrating 100-fold increase in tissue concentration of gemcitabine compared to IV delivery of gemcitabine at the same infusion rate.
| 14 |
We concluded that TAMP can achieve drug penetration into the surrounding tissue and can achieve high dose concentrations in local tissue. The tissue concentration with IV and/or distant from TAMP site (likely after recirculation through systemic system) were two orders of magnitude lower than tissue levels achieved with TAMP (p<0.02).
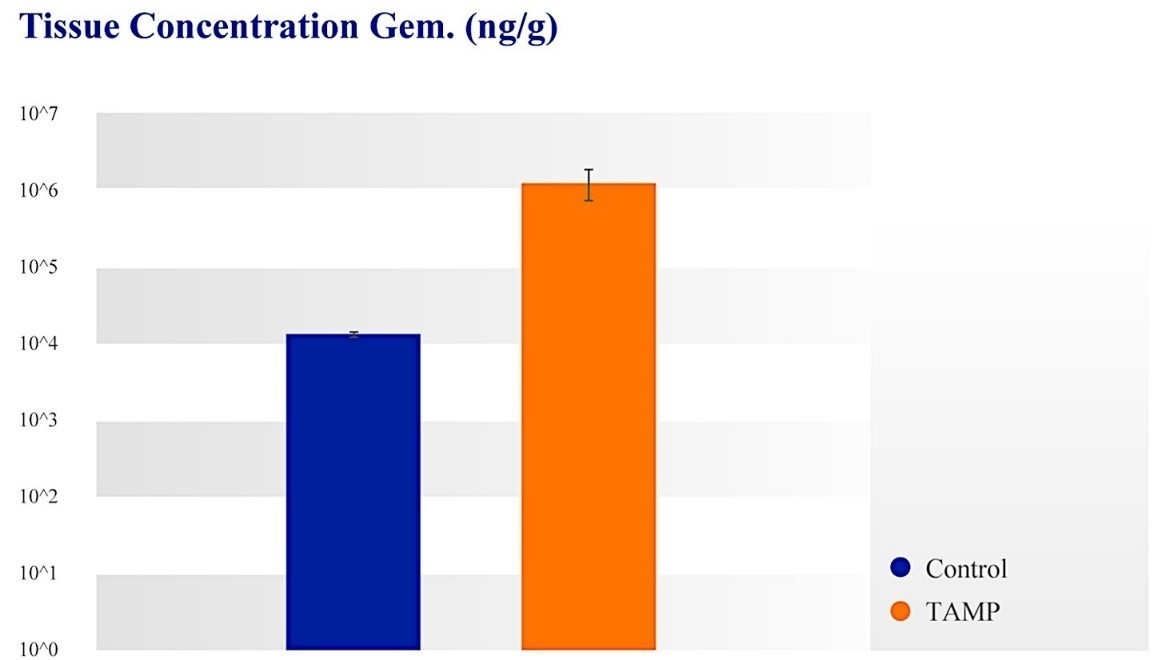
Figure 11: Local tissue concentration of gemcitabine. control (Blue): Intravenous infusion versus TAMP (Orange): TAMP: IA infusion. The tissue concentration with intravenous infusion and/or distant from TAMP site (likely after recirculation through systemic system) are 100-fold lower than tissue levels achieved with TAMP.
This animal lung study successfully validated the ability of RenovoCath to deliver small molecules locally and effectively to lung tissue.
III. Based on the results of preclinical studies, increase in local tissue delivery of gemcitabine in LAPC may enhance tumor reduction and therapeutic response
In relevant mouse models of pancreatic tumors, it has been demonstrated that targeted IA infusion of gemcitabine into the pancreas after surgical isolation of arterial blood flow has a superior therapeutic effect with greater reduction in tumor volume compared to the same concentration administered by conventional IV injection. To achieve a comparable reduction in tumor growth as seen with IA treatment, gemcitabine had to be given intravenously at over 300 times the dose which was associated with increased toxicity.
TAMP and Radiation
Traditionally the goal of radiation includes debulking the tumor and/or acting as a chemo-sensitizer. In our RR1 dose escalation safety study and RR2 observational registry study, the benefit of TAMP appeared to be enhanced in patients with prior radiation. As we were observing this effect months after radiation and although several randomized studies have not demonstrated a benefit of chemotherapy plus radiation versus chemotherapy alone, we hypothesized that a direct effect of radiation on the vasculature may be enhancing the effect of TAMP. One of the side effects of radiation is a decrease in the micro-vasculature in the irradiated tissue including the small blood vessels that exist in the vessel walls themselves. Therefore, we postulated that by eliminating microvasculature in and around the vessel wall, radiation may enhance drug penetration into the tissue via TAMP (Figure 12). As such, a possible enhancing effect of radiation on TAMP may involve decreasing washout of the drug as it crosses the arterial wall by preventing draining into the surrounding microvasculature.
| 15 |
We completed a pig study where we observed the impact of TAMP in recruiting the vasa vasorum (small blood vessels within the larger blood vessel walls) around the vessel during drug/dye infusion. It was discovered that the dye drained into the vasa vasorum and other small vessels in the adjacent tissue (Figure 12); as these vessels can directly connect to the adjacent venous system, the microvascular networks can serve as an “escape route” for drugs. Ultimately this direct washout can reduce the amount of drug concentration in the tissue. Radiation pretreatment may enhance the impact of TAMP by attenuating this escape route.
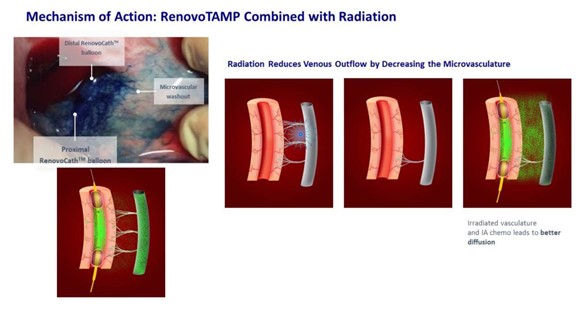
Figure 12: Mechanism of TAMP and radiation reduces venous outflow by decreasing the microvasculature networks that could act as an “escape route” for the drugs. The photo on the left illustrates this effect in a dye infusion study in the porcine animal model. The panel on the right demonstrates venous chemotherapy washout without radiation versus less venous escape routes for chemotherapy following radiation.
| 16 |
We further advanced this theory by conducting a pig study to directly test whether radiation can enhance tissue uptake by TAMP. In a single-animal study, we examined the use of SBRT pre-treatment on one leg followed by TAMP versus TAMP without prior radiation therapy on the opposite leg. The leg of the animal that was pre-treated with radiation demonstrated more pronounced tissue staining with methylene blue dye and increased gemcitabine concentration via punch biopsy. Based on these findings, we believe that the benefit of prior radiation on clinical outcomes with TAMP may be improved by the effect of radiation on microvasculature between the vessel wall and the tumor.
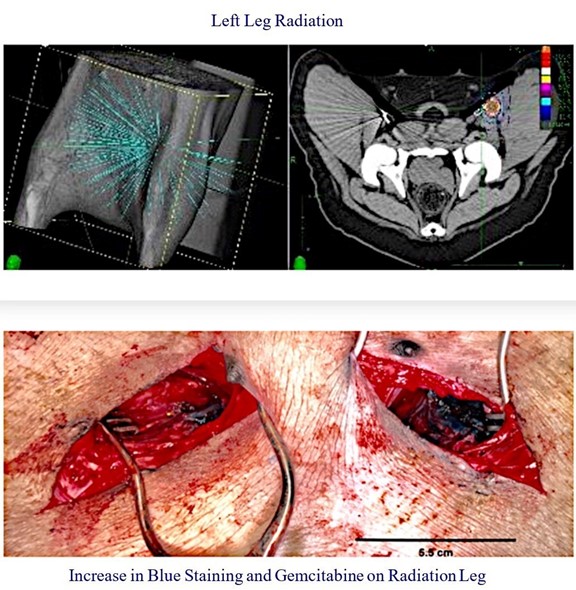
Figure 13: To demonstrate the effect of radiation pre-treatment, we delivered radiation therapy to the left leg of a pig. After waiting one month for the therapy to fully affect the vasculature, we performed TAMP on the left and right leg arteries with blue dye and gemcitabine. Dissection revealed better dye penetration into the tissue on the left (irradiated) leg, and punch biopsy demonstrated higher gemcitabine concentration in the left leg.
We have demonstrated that the TAMP therapy allows targeted small molecule drug delivery into the tissue surrounding the vessel wall, without need to identify tumor feeder blood vessels. The mechanism of action is the exclusion of distal (downstream) and side branch vessels in the isolated segment and creating a pressure gradient by infusing the drug over time. The pressure gradient results in a diffusion-mediated delivery of drug into the surrounding tissue. With the use of gemcitabine, the procedure appears safe in terms of local toxicity in the vasculature. Using this approach, we can achieve increased drug delivery into the surrounding tissue in the range of 4 cm-tissue penetration as well as concentration orders of magnitude larger than what can be achieved with IV infusion. Lastly, TAMP appears to be enhanced by prior radiation of tissue, possibly by decreasing the microvasculature and subsequent potential chemotherapy washout.
| 17 |
LAPC Clinical Development
TAMP has been studied in a Phase I/II dose-ranging study of 20 subjects with LAPC (RR1) and in an observational study that enrolled 25 additional subjects with pancreatic cancer (RR2); two subjects from the RR1 safety study continued to receive treatment in the RR2 observational registry study. We subsequently launched a Phase III registration trial (TIGeR-PaC). On March 8, 2023, we announced interim analysis results of the study suggesting a 6-month potential improvement in median overall survival with RenovoGem, pending ongoing clinical investigation. We believe this first-of-two interim analyses indicates that the TIGeR-PaC study is on track to demonstrate increased lifespan for patients being treated with RenovoGem for LAPC. Final analysis will be conducted after 86 protocol-specified events have occurred in the SBRT population with two planned interim analyses: this first analysis with 30% of the specified events (deaths) reported and the second analysis when 60% of the events have been reported (expected in 2024).
Phase I/II Dose-Ranging Study: RR1
Study Design
A Phase I/II safety study of our TAMP therapy has been completed in subjects with LAPC (Phase I/II RenovoCath/Gem RR1). This multicenter, prospective, open label, interventional, nonrandomized, intra-subject dose escalation study evaluated IA gemcitabine delivered locally to the pancreas using RenovoCath in 20 subjects with LAPC. The primary objectives of the study were (1) to establish the maximum tolerated dose (“MTD”) and (2) to study the safety and tolerability of IA gemcitabine administered by RenovoCath at doses ranging from 250 mg/m2 to 1000 mg/m2. Secondary endpoints included overall survival, CA 19-9 marker change, change in tumor size based on RECIST 1.1 (Response Evaluation Criteria in Solid Tumors) criteria, and pain scores and narcotic use. Adverse events were collected from the first IA gemcitabine infusion until 3 months following the final IA gemcitabine infusion. Subjects were followed for survival.
Treatment constituted introducing RenovoCath to target vessel (adjacent to tumor) via catheterization, occluding the targeted segments via the RenovoCath balloons, and infusing gemcitabine in the occluded segment. To minimize ischemia (damage due to cessation of blood flow) the infusion was limited to 20 minutes and an anticoagulant (heparin) was given during the procedure. Tissue markers were followed post procedure to ensure lack of local tissue damage-toxicity (AST, ALT, Lipase and Amylase).
Treatment was administered in four 28-day cycles, each of which consisted of two IA doses of gemcitabine, one on day 1 and one on day 15, with a two-week rest period between cycles. The first six subjects received a starting dose of 250 mg/m2, and doses increased by 250 mg/m2 in each subsequent cycle culminating with the full dose of 1,000 mg/m2. After the initial six subjects, the starting dose increased to 500 mg/m2 for one cycle, after which dosing increased to 750 mg/m2 for the second cycle, and then the full 1,000 mg/m2 dose for the remaining 2 cycles. Each subject underwent CT scanning prior to the first procedure for the selection of the optimal target vessel most proximal to the tumor.
Study Subjects and RenovoGem Exposure
The median age of subjects was 66.7 years with a gender distribution of 9 men and 11 women. Prior treatment included chemotherapy and radiation therapy in 6 (30%), chemotherapy alone in 5 (25%) and no prior therapy in 9 (45%) subjects. Collectively the 20-subject cohort received 101 IA treatments. It is important to note that 9 of the 20 subjects had a biliary stent or drain in place before the first IA procedure.
Trial Results
Safety
There was no evidence of local tissue toxicity in any patients post procedure as measured by liver and pancreatic enzymes. Out of 101 procedures, adverse events were reported in 11 subjects, including catheterization/procedure-related events with arterial dissections at treatment sites (3), pseudoaneurysm in a visceral artery (1), complications away from the treatment site and site complications (2).
| 18 |
Serious adverse events were reported in 9 subjects during the study. Overall survival (including deaths that occurred following disease progression) was followed in all study subjects. The number of subjects with serious adverse events is shown in the table below.
Summary of Serious Adverse Events for 9 subjects in RR1 Dose Ranging Study
| Serious Adverse Event | N=20 | |
| Cardiac Arrest | 1/20 (5%) | |
| Dehydration | 1/20 (5%) | |
| Duodenal obstruction | 1/20 (5%) | |
| Gastritis | 1/20 (5%) | |
| Infection | 1/20 (5%) | |
| Intraoperative arterial injury-dissection | 3/20 (15%) | |
| Intraoperative arterial injury-lower extremity | 1/20 (5%) | |
| Pain-Abdominal NOS | 1/20 (5%) | |
| Respiratory failure | 1/20 (5%) | |
| Sepsis | 3/20 (15%) | |
| Neutropenia | 4/20 (20%) |
This table shows serious adverse events reported in 9 of the 20 subjects during the study. Several subjects had more than one serious adverse event.
Efficacy
The principal evaluation of efficacy was survival. All subjects were followed for survival after the end of IA gemcitabine treatment. All subjects have died, with the longest having an overall survival of 35.9 months.
Subjects Who Received More IA Treatment Cycles Survived Longer
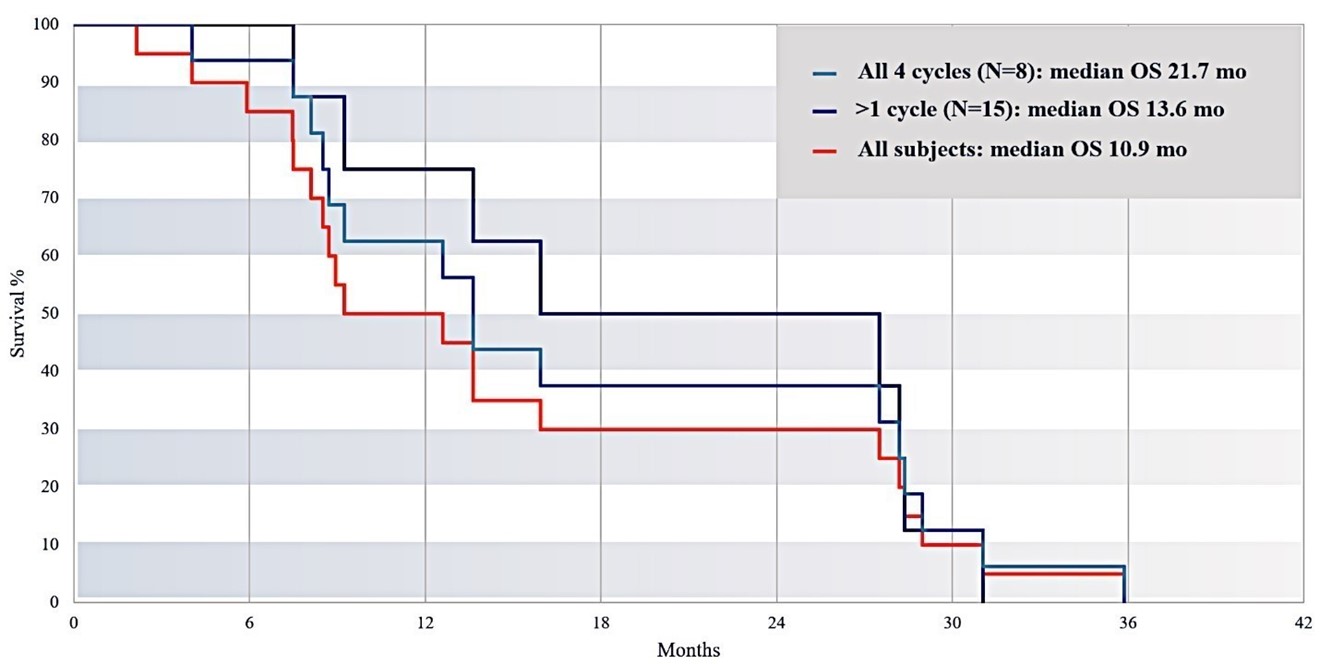
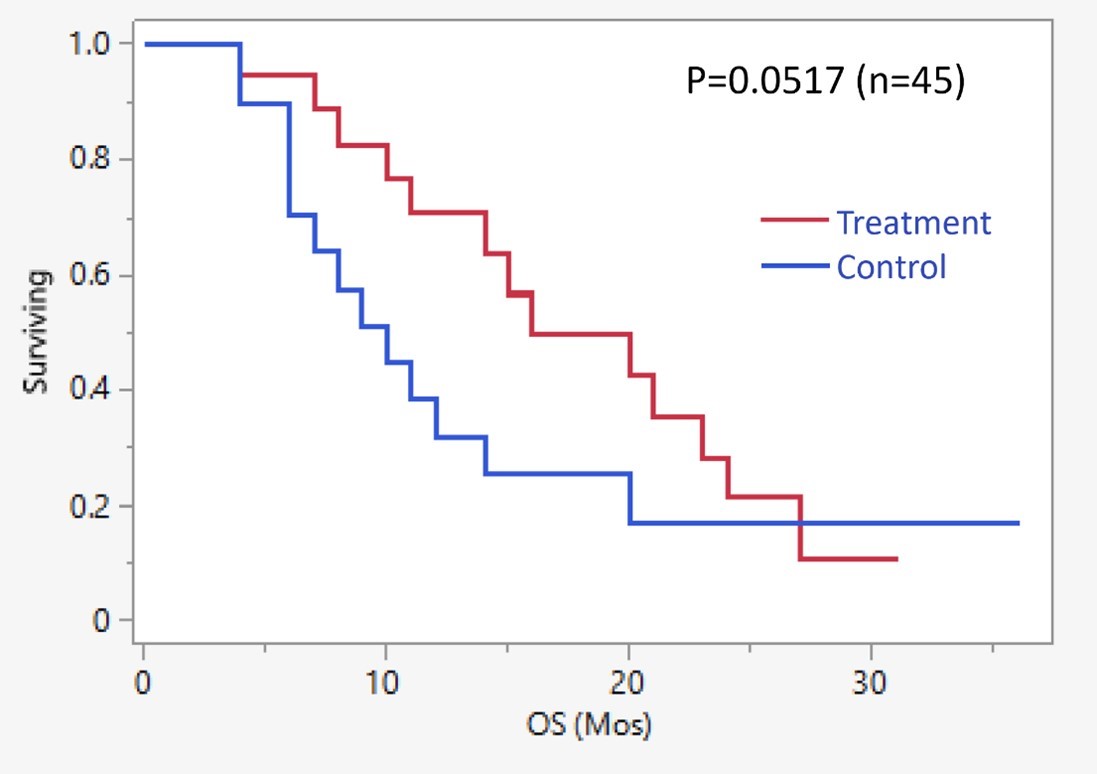
Figure 14: This chart shows survival as a function of total number of IA treatment cycles received. Subjects receiving all 4 cycles (n=8) had a median survival time of 21.7 months, compared to a median survival time of 10.9 months for all subjects (n=20).
| 19 |
Subjects Who Received Greater Cumulative Exposure to RenovoGem Survived Longer
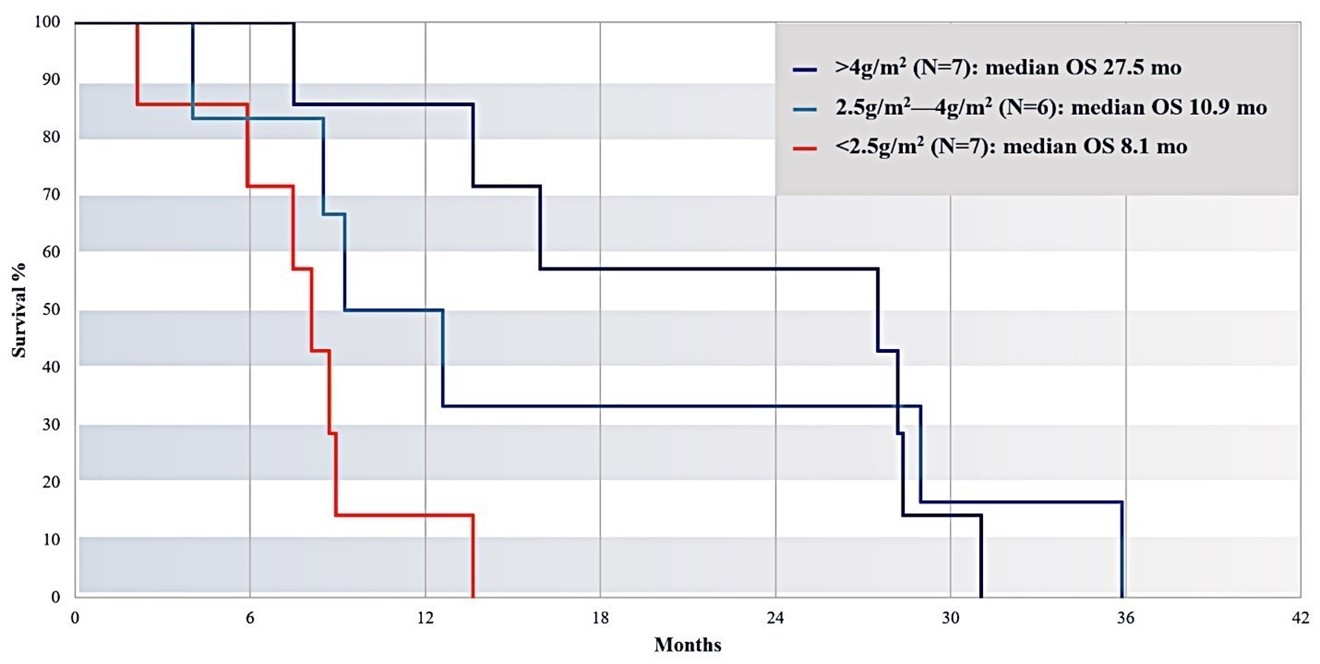
Figure 15: Splitting the entire cohort into equal tertiles based on total dose received, patients receiving the lowest total dose (<2.5g/m2; n=7) demonstrated the lowest median overall survival (8.1 months) compared to patients in the group receiving the next higher total dose (>2.5g/m2, but <4g/m2; n=6; median OS=10.9 months), and patients in the group receiving the highest total dose (>4g/m2; n=7; median OS=27.5 months).
Subjects Who Had Prior Radiation Exposure Survived Longer Than Those Who Did Not
As shown below in Figure 16, fifteen subjects received more than 1 cycle of IA gemcitabine treatment. The red line represents subjects without any prior treatment or received prior chemotherapy only (n=10; median OS=13.6 months). The dark green line depicts subjects who received prior chemoradiation (n=5; median OS=28.2 months). P < 0.05 for survival between the two subsets. Survival appeared to be longer in subjects who had prior chemoradiation.
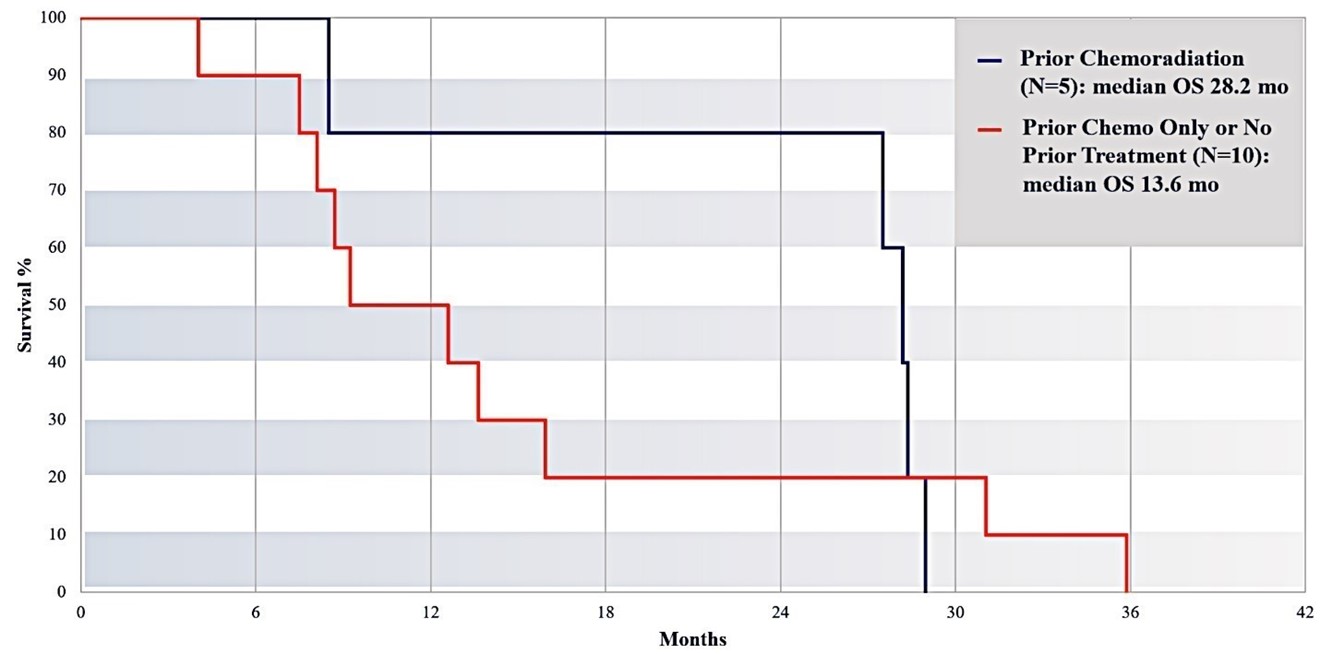
Figure 16: Survival of subjects with or without prior chemoradiation. Subjects with prior chemoradiation (n=5) had a median survival time of 28.2 months, compared with a median survival time of 13.6 months for subjects without prior chemoradiation (n=10).
| 20 |
Disease Progression Based on RECIST 1.1 Criteria
The RECIST 1.1 criteria was used to compare the baseline and follow up CT images submitted by sites. Follow-up CT scans obtained 5 months after initiation of IA gemcitabine therapy were submitted for 17 of the 20 subjects and were compared to the baseline images. Six of the 17 subjects (35.3%) experienced tumor progression, 1 (5.9%) had a partial response, and 10 (58.8%) demonstrated stable disease 5 months post treatment initiation. Two of the 6 subjects with tumor progression received less than 1 cycle (only 1 treatment) of IA gemcitabine. Among 15 subjects who received more than 1 cycle (2 treatments), 26.7% had disease progression, 6.7% had partial response and 66.7% had stable disease 5 months post IA therapy.
CA 19-9 Tumor Marker Change
CA19-9 is a protein that can be detected in serum and is a biomarker of pancreatic cancer; its levels can be used to assess tumor response to therapy. Twelve of 20 subjects had measurable CA 19-9 tumor markers. The final CA 19-9 tumor marker levels were lower in 7 of 12 (58%) and greater in 5 of 12 (42%) subjects. It is notable that final tumor marker levels were lower in 4 of 5 subjects with prior chemoradiation and higher in 5 of 7 subjects without prior chemoradiation.
Observational Registry Study RR2
We launched the RR2 observational registry study in January 2016 to further explore the clinical utility of the TAMP procedure. The key inclusion criteria were patients with locally advanced or borderline resectable pancreatic adenocarcinoma confirmed by histology or cytology. This was an observational patient registry study with endpoints of safety and survival following IA gemcitabine treatment with RenovoCath. The study was conducted at 7 sites in the US and subsequently closed on August 2019 except for one US site (that did not participate in the Phase III study). This last site in the study was officially closed in September 2020. Over the 3 years that the trial was open, we enrolled 25 subjects with LAPC. Two of those subjects had participated in our Phase I/II RenovoCath/Gem RR1 trial: each received 8 IA gemcitabine infusions prior to enrollment in the RR2 study. A summary of data updated through January 2021 is presented below.
The study initially enrolled LAPC subjects without regard to prior radiation or chemotherapy. In April 2017, after the observation of longer survival of subjects with prior chemoradiation versus subjects who had not had prior radiotherapy, entry into the registry was restricted to subjects with LAPC who had received prior radiation. Of note, one subject who had prior pancreatic cancer surgery (Whipple procedure) would not normally have been enrolled in the study but was included for safety observations as her physician had previously planned IA gemcitabine therapy.
Investigators in the study reported all Serious Adverse Events from the first IA gemcitabine infusion to at least 60 days after their last procedure, but reporting of non-serious adverse events was optional. All subjects received gemcitabine 1000 mg/m2 every two weeks, except one who received 500 mg/m2, typically for a total of 8 doses. Subjects were followed post-treatment for survival.
Study Subjects and RenovoGem Exposure
Twenty-five subjects were enrolled at 7 sites. The study enrolled 15 women (60%) and 10 men (40%); with a mean and median age of 73. Of the 25, 10 (40%) had no prior therapy, 8 (32%) had prior radiotherapy and chemotherapy, 6 (24%) had prior chemotherapy alone and 1 (4.5%) had surgery (Whipple procedure).
Two subjects were continuations from the previous Phase I/II RenovoCath/Gem RR1 study, and as a result, received more than eight treatments (total in both studies). The treatment received summary is shown in the table below:
Dosing Treatments for RR2 Observational Registry Study, for 25 Subjects Enrolled at 7 Sites
| Number of Dosing Treatments | N=25 | |
| 1 | 5/25 (20%) | |
| 2 | 3/25 (12%) | |
| 3 | 4/25 (16%) | |
| 4 | 5/25 (20%) | |
| 6 | 2/25 (8%) | |
| 7 | 2/25 (8%) | |
| 8 | 2/25 (8%) | |
| >8 | 2/25 (8%) |
| 21 |
Twenty-five subjects, 15 women (60%) and 10 men (40%); with a mean and median age of 73 were enrolled at 7 sites. Of 25, 10 (40%) had no prior therapy, 8 (32%) had radiotherapy and chemotherapy, 6 had chemotherapy alone and 1 (4.5%) had surgery (Whipple procedure).
In the 25 patients, 109 total IA treatments were administered through one or more of the following arteries:
| ● | Common Hepatic Artery | |
| ● | Splenic Artery | |
| ● | Celiac Axis | |
| ● | Superior Mesenteric Artery |
Trial Results
Safety
There were a number of adverse events reported. The most common were nausea (36%), vomiting (28%), abdominal pain (32%), followed by vascular access complications (16%). The less common adverse events reported (< 5%) included rash, allergic reaction, retroperitoneal hemorrhage, sepsis, ischemic bowel, arterial spasm, atrial fibrillation, chest pain, back pain, hypoglycemia, pruritis, and other GI issues. No deaths were noted in the immediate post-treatment period. No deaths were considered related to study treatment. Survival is summarized as an efficacy evaluation.
Summary of Key Safety Observations
| ● | Neither pancreatitis nor local tissue toxicity was reported in LAPC subjects without prior surgery. | |
| ● | There was no instance of arterial dissection in this study. | |
| ● | The incidence of sepsis was lower in this study (1/25 subjects receiving 94 infusions) compared with the incidence in Phase I/II RenovoCath/Gem RR1 (3/20 subjects receiving 101 infusions). The subject with sepsis did not have a biliary stent or drain and the source of the sepsis was not identified. No sepsis events were noted after 51 infusions in 12 subjects with biliary stents, who received peri-procedure antibiotics. The incidence of sepsis in the RR2 observational registry is like that of pancreatic cancer subjects receiving myelosuppressive chemotherapeutic regiment in other studies. |
Efficacy
Excluding the subjects with prior or post pancreatic cancer surgery, median survival (n=22) from the time of first IA gemcitabine treatment was 5.43 months, as illustrated in Figure 16 below, whereas median overall survival (from date of diagnosis) was 13.0 months (Figure 17).
| 22 |
Survival of all Subjects from first IA Gemcitabine Treatment (Median 5.43 Months)
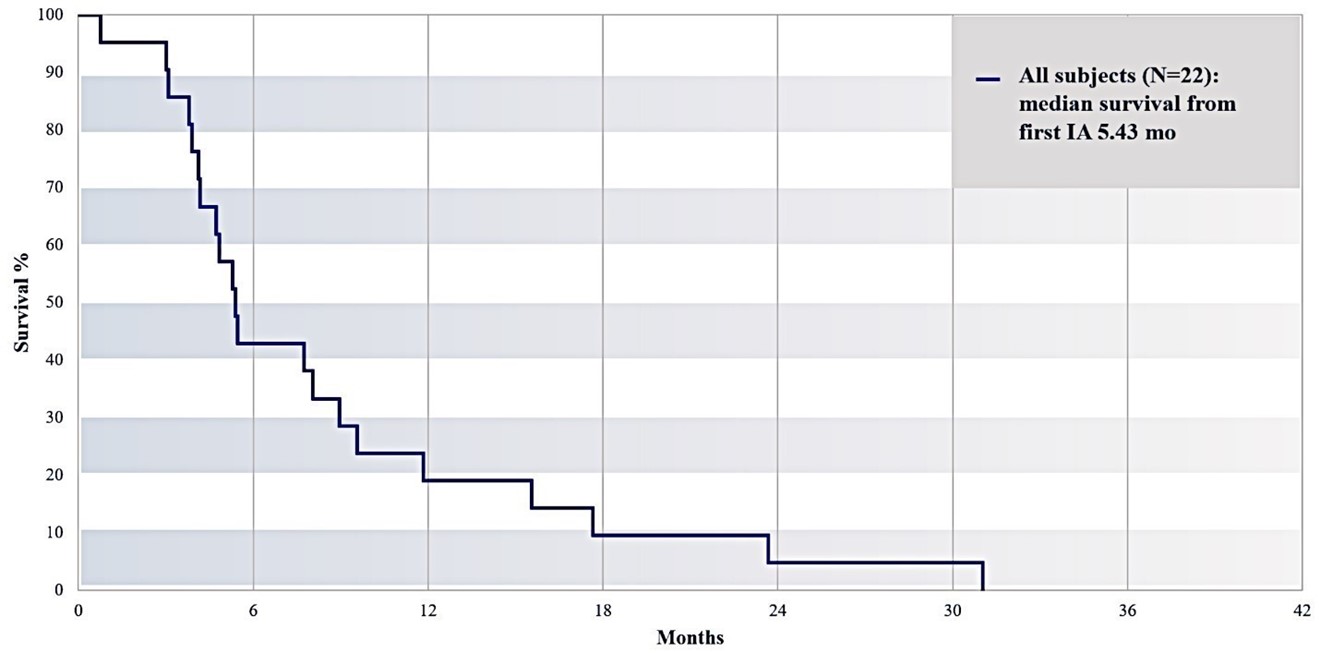
Figure 17. Overall RR2 observational registry study cohort (N=22) survival from first IA treatment until date of death.
Overall Survival of All Subjects (Median Overall Survival 13 Months)
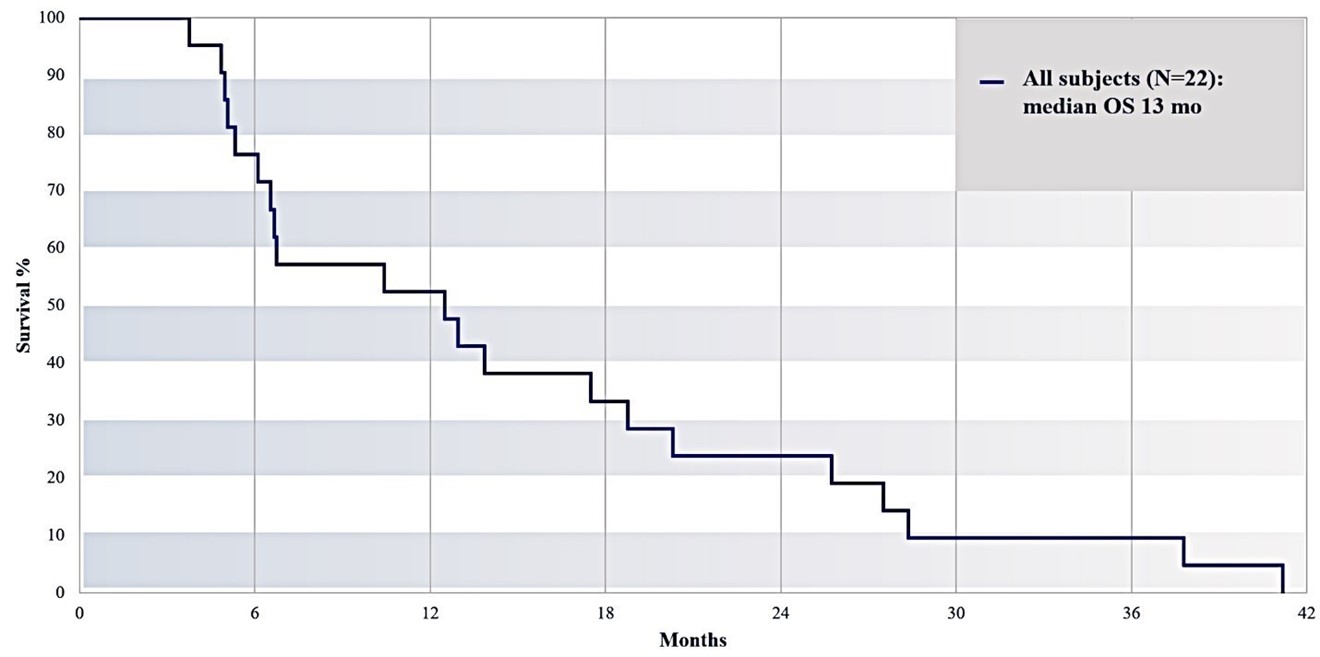
Figure 18: Overall RR2 observational registry study cohort (N=22) overall survival from date of diagnosis.
As in Phase I/II RenovoCath/Gem RR1, subjects with prior radiation and chemotherapy demonstrated longer survival than other subjects.
| 23 |
Subjects with Prior Chemoradiation Survived Longer than Subjects with Prior Chemotherapy Only
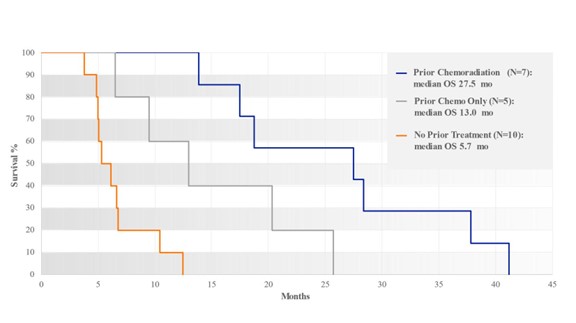
Figure 19: Survival as function of previous treatment received for RR2 registry study subjects. As in RR1 study, subjects with prior radiation and chemotherapy demonstrated longer survival than other subjects.
In summary, the results of the RR2 observational registry build on the findings of the Phase I/II RenovoCath/Gem RR1 study. The use of RenovoCath in this patient population can be undertaken with adequate safety, with adequate attention to procedural technique including careful use/manipulation of a guide catheter to prevent arterial dissection, and with the administration of peri-procedure antibiotics in patients with prior biliary stent/drain.
RR1 and RR2 Conclusions
Patients with LAPC treated with TAMP showed efficacy signals:
| ● | Survival of patients with LAPC following TAMP was similar to that observed in the previous Phase I/II RenovoCath/Gem RR1 study. | |
| ● | Patients with biliary stents or drains who received TAMP who received prophylactic peri-procedure antibiotics experienced no episodes of sepsis. | |
| ● | LAPC patients who received prior radiation and chemotherapy had longer survival than those without prior radiotherapy. | |
| ● | In the RR2 observational registry study, treatment via the Superior Mesenteric Artery (SMA) showed the greatest survival benefit. It is believed that this is a result of the high contact area between the SMA and the tumor tissue. | |
| ● | The registry study (RR2) results combined with the Phase I dose escalation study (RR1) further validates prior radiation and treatment location as predictors of overall survival (in combination, RR1 and RR2 data were statistically significant for these two variables). |
Based on the FDA’s safety review of our Phase I/II study and clinical outcome, the FDA allowed us to proceed to evaluate RenovoGem within our Phase III registrational clinical trial.
| 24 |
TIGeR-PaC Phase III Trial (RR3)
With completion of RR1 and RR2, we obtained FDA approval for Phase III IND study in February 2018 comparing TAMP with IA gemcitabine to standard of care. In the FDA pre-IND meeting, the FDA confirmed the study design and endpoints and indicated that this Phase III study should result in New Drug Application approval if successful. In April 2018, we obtained Orphan Drug Designation for the use of RenovoGem in patients with pancreatic cancer. Depending on the progress of the trial and the potential observed benefit of RenovoGem, we will evaluate submitting a request to the FDA for Breakthrough Therapy Designation.
The primary endpoint of the study is overall survival, from time of randomization until death. Secondary endpoints include but not limited to progression free survival and quality of life questionnaire results. The study is a multi-center, open-label, randomized active-controlled study of subjects with locally advanced pancreatic adenocarcinoma which is unresectable according to NCCN guidelines. The study is currently enrolling patients in the US.
The study design is as follows: all patients receive a four-month induction phase of IV chemotherapy and radiation prior to randomizing to 4 cycles (8 treatments) of TAMP or 4 cycles of continuation of IV chemotherapy. In December 2021, we amended the protocol for our Phase III clinical trial to only allow for SBRT radiation during the induction phase of the study. We had previously permitted both SBRT and IMRT. Patients receiving IMRT were required to complete 25 treatments prior to being randomized into our study. In comparison, patients receiving SBRT are only required to complete 5 treatments. IMRT is generally less tolerable than SBRT, and we had observed a higher drop out for patients on IMRT. While TAMP data versus historical controls predicts a much greater survival benefit, the TIGeR-PaC study is powered to detect a 6-month survival benefit.
A study flowchart is shown below. Subjects with stable or responding disease after approximately 4 months in induction therapy and who are not surgical candidates will then be randomized 1:1.
TIGeR-PaC Study Flowchart with Chemoradiation Induction Phase
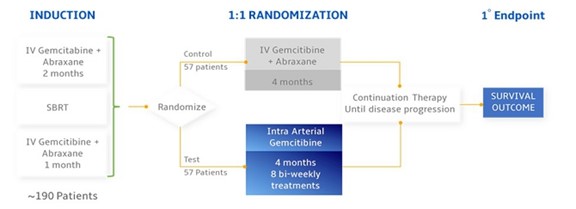
Figure 20: TIGeR-PaC Study Flowchart. All subjects undergo a 4-month induction phase that includes IV gemcitabine + Abraxane and radiation therapy. If the subjects are stable with LAPC post-induction, they are randomized 1:1 into control group (IV gemcitabine + Abraxane) versus treatment group (IA gemcitabine via TAMP therapy). Subjects are then administered continuation therapy until disease progression and followed through survival.
In December 2021 we amended the protocol for this clinical trial to only allow for SBRT during the induction phase of the study (prior to randomization). We had previously permitted both SBRT and IMRT. Patients receiving IMRT must complete 25 radiation treatments in combination with oral chemotherapy during the induction phase of the study, which takes between 35 and 56 days to complete. In comparison, patients receiving SBRT during the induction phase are only required to complete 5 treatments, over 5 consecutive days, and do not receive oral chemotherapy. The decision to modify the study population was based on the observation in the Phase III TIGeR-PaC study that IMRT patients had a higher dropout rate during the induction phase of the study due to the high frequency of hospital visits and side effects from the required concurrent chemotherapy. As part of the pre-randomization, induction phase change made to the protocol, we initiated a review of the statistical considerations for the study and in June 2022, submitted the Modified SAP to FDA. As part of the Modified SAP, we now plan to (i) analyze only patients receiving SBRT, consistent with the protocol change made in December 2021, (ii) include a second interim analysis, (iii) change the total number of SBRT patients randomized in the study to 114 (a reduction from the original 200 patients) with a total of 86 deaths from SBRT patients, including all deaths from SBRT patients enrolled in the study before the submission of the Modified SAP, and (iv) repower the study from 90% to 80%, which is commonly used in clinical trials. We believe these changes will shorten the timeframe needed to complete the study and also significantly decrease our costs. We have not discussed the protocol amendment or the Modified SAP with the FDA, and we cannot provide any assurance that the FDA will agree with these modifications. On March 8, 2023, we announced interim analysis results of the study suggesting a 6-month potential improvement in median overall survival with RenovoGem, pending ongoing clinical investigation. We believe this first-of-two interim analyses indicates that the TIGeR-PaC study is on track to demonstrate increased lifespan for patients being treated with RenovoGem for LAPC. Final analysis will be conducted after 86 protocol-specified events have occurred in the SBRT population with two planned interim analyses: this first analysis with 30% of the specified events (deaths) reported and the second analysis when 60% of the events have been reported (expected in late 2024), with the final study readout in 2026.
| 25 |
In this first interim analysis, the control and treatment arms demonstrated divergence in median overall survival for patients. The study is designed to randomize 114 patients (57 in each arm) with all patients receiving upfront induction chemotherapy and SBRT. The TIGeR-PaC DMC met and determined the interim data is promising and warrants continuation of this trial. As of the date of the analysis, 45 patients from U.S. sites had been randomized in this trial and the survival status of all subjects was used for the analysis.
| ● | 23 patients were randomized to IA gemcitabine (RenovoGem investigational treatment) arm and 22 to continuation of IV gemcitabine and nab-paclitaxel (control or standard of care) arm. There were an equal number of primary events, 13 in each arm. | |
| ● | The median overall survival in the IV gemcitabine and nab-paclitaxel control arm was 10 months, versus 16 months in the IA RenovoGem arm. (NOTE: Both arms’ median overall survival calculations do not include 4 to 5-months of life since diagnosis during the induction chemotherapy and radiation phase of the trial). | |
| ● | Observed a positive trend in median overall survival by 24-weeks (6-months); in this interim analysis, the statistical significance was not reached to stop the study early (p=0.051) |
Clinical Pharmacokinetic (PK) Data in Patients with LAPC Treated with Gemcitabine via TAMP
We expect IA gemcitabine delivered via the TAMP technique to have a pharmacokinetic profile that is distinct from intravenous gemcitabine dosing. Furthermore, with local delivery of gemcitabine into the tissue via TAMP and drainage into the liver prior to systemic circulation, we anticipate lower systemic levels of gemcitabine.
We presented the initial results of this substudy at ASCO-GI 2023 in San Francisco, CA. At the time of the analysis, 13 samples were collected across 5 TIGeR-PaC clinical sites. We demonstrated that the cohort had an average greater than 50% reduction in systemic drug exposure with IA delivery of gemcitabine using TAMP when compared with IV administration.
Second Solid Tumor Indication — eCCA
eCCA Overview
Cholangiocarcinoma is the second most common primary malignant tumor of the liver with over 7,000 new cases diagnosed annually in the U.S. Cholangiocarcinoma, or liver cancer (“CCA”) develops after malignant transformation of the biliary tract mucosa. According to Cholangiocarcinoma (CCA) - Market Insight, Epidemiology And Market Forecast, the global market of CCA was estimated to be $786.1 million in 2021 and the U.S. accounted for $237.3 million. Furthermore, the market size for global CCA therapeutics is estimated to grow by $83 million during 2019-2023 with a compound annual growth rate of 6%. Advanced age, male gender, primary sclerosing cholangitis (PSC), inflammatory bowel disease, pancreatitis, and cirrhosis are some predisposing factors for development of CCA.
Based on the tumor location CCA is defined as intra-hepatic, or within the liver, or extrahepatic, or outside the liver. The eCCA subset of CCA patients is about 3,000 cases per year. eCCA is a disease with an exceptionally poor prognosis.
| 26 |
eCCA Current Treatment Landscape and Limitations
Most patients with eCCA have localized disease with possible extension of the tumor around the bile duct. Based on local extension of the disease, treatment options include surgery, chemotherapy, and radiation therapy. Surgical resection offers the only chance for curative therapy for patients with eCCA; however, the surgery is associated with high mortality and morbidity and most patients are not candidates. Systemic chemotherapy is a primary mode of treatment in these patients as a form of palliation, which is associated with morbidity and limited improvement in survival.
Current standard chemotherapy treatment in these patients is based on the ABC-2 Trial: a randomized trial of 410 patients with unresectable CCA (the study included intrahepatic, within the liver, and extrahepatic cholangiocarcinoma patients). Patients were treated with gemcitabine plus cisplatin, consisting of a three-week cycle, with treatments on Days 1 and 8 and dosing of gemcitabine at 1000 mg/m2 and cisplatin at 25 mg/m2. Reported median overall survival for patients on such a regimen (11.7 months) was greater than for patients receiving gemcitabine alone (8.1 months). With this standard of care treatment, commonly observed Grade 3-4 toxicities include anemia, leukopenia, neutropenia, thrombocytopenia, lethargy, nausea/vomiting, and anorexia. In the ABC-02 trial the efficacy of gemcitabine/cisplatin combination was not significantly different from that of gemcitabine alone in patients with extrahepatic cholangiocarcinoma. For this reason, a standard of care practice has not been established for extrahepatic cholangiocarcinoma.
More recently, the Phase III TOPAZ-1 study was presented at the 2022 ASCO GI in San Francisco, CA and demonstrated that the addition of durvalumab to the previously described gemcitabine/cisplatin regimen increased median overall survival from 11.6 months to 12.8 months in this patient population and subsequently received FDA approval.
RenovoGem for the Treatment of eCCA
Similar to RenovoGem for LAPC, we believe that RenovoGem may overcome the current treatment limitations of eCCA. In this setting, patients with eCCA have several tumor characteristics that create the potential for RenovoGem to be more effective than systemic chemotherapy. These characteristics include:
| ● | Local disease with possible extension of disease to local vasculature; | |
| ● | Avascular nature of the tumor lends itself to our TAMP approach, overcoming the limitations in drug delivery by targeting the periductal proper hepatic artery or left or right hepatic artery; | |
| ● | Gemcitabine, used as a target molecule for this tumor type, has already been demonstrated to be safe in terms of local toxicity targeted via our approach to this vasculature and organ; and | |
| ● | The bile duct around the hilum is usually within 1-14 mm (mean of 3.8 mm) of the hepatic artery: a reasonable target for TAMP therapy given the potential 4 cm tissue penetration of drug. |
Clinical Development of RenovoGem in eCCA
Rationale
In May 2020, the FDA granted us Orphan Drug Designation in May 2020 for RenovoGem for the treatment of CCA. We are planning to evaluate RenovoGem in a second indication in a Phase II/III trial in extrahepatic (or outside the liver) cholangiocarcinoma (or eCCA), cancer that occurs in the bile ducts that lead out of the liver and join with the gallbladder. After significant input from key opinion leaders across the spectrum of relevant medical specialties and feedback from the FDA, we submitted the protocol for a Phase II/III eCCA clinical trial to FDA, and after receiving feedback, we are finalizing the protocol and also including incorporating a recently approved drug, durvalumab into the study protocol with guidance from the Steering Committee. We have also secured FDA Orphan Drug Designation for RenovoGem for the treatment of cholangiocarcinoma, which would provide us with seven years of orphan exclusivity to market RenovoGem for our eCCA indication upon NDA approval, provided that we are the first sponsor to obtain FDA approval for IA gemcitabine for the eCCA indication.
| 27 |
Market Opportunity
We are currently developing RenovoGem for LAPC. We also intend to develop it for eCCA, and potentially for locally advanced lung cancer, locally advanced uterine cancer and glioblastoma. We estimate that the total annualized addressable market opportunity for RenovoGem for our first market, LAPC, in the United States is approximately $0.5 billion and globally could exceed $1 billion based on a third-party market research analysis. The total cost of care for a patient on the standard of care treatment of gemcitabine plus Abraxane is estimated at $67,216, which if applied to 60,000 pancreatic cancer cases per year would total $4 billion per year for the total US pancreatic cancer market.
Beyond our initially targeted subset of LAPC patients, we see potential to evaluate RenovoGem in additional settings where it may help to get more patients to surgery, prolong life, enhance systemic therapy or provide local therapy with fewer side effects than alternative treatments. These may include patients with stage 1 or stage 2 pancreatic cancer receiving neoadjuvant as well as in subpopulations of patients with metastases who also have locally advanced disease. Based on third-party market research, several physicians mentioned the role for local therapy as an adjunct for systemic chemotherapy as well as for patients who decline systemic chemotherapy. Among the locally advanced (stage 3) patients diagnosed with the cancers shown in Figure 21, we estimate that approximately125,000 could be potentially addressable via RenovoGem.
Below is published epidemiology data showing the 2023 estimated annual incidence of the following tumor types in the United States to be greater than 350,000 patients in the aggregate.
Potential Market Opportunity for RenovoGem
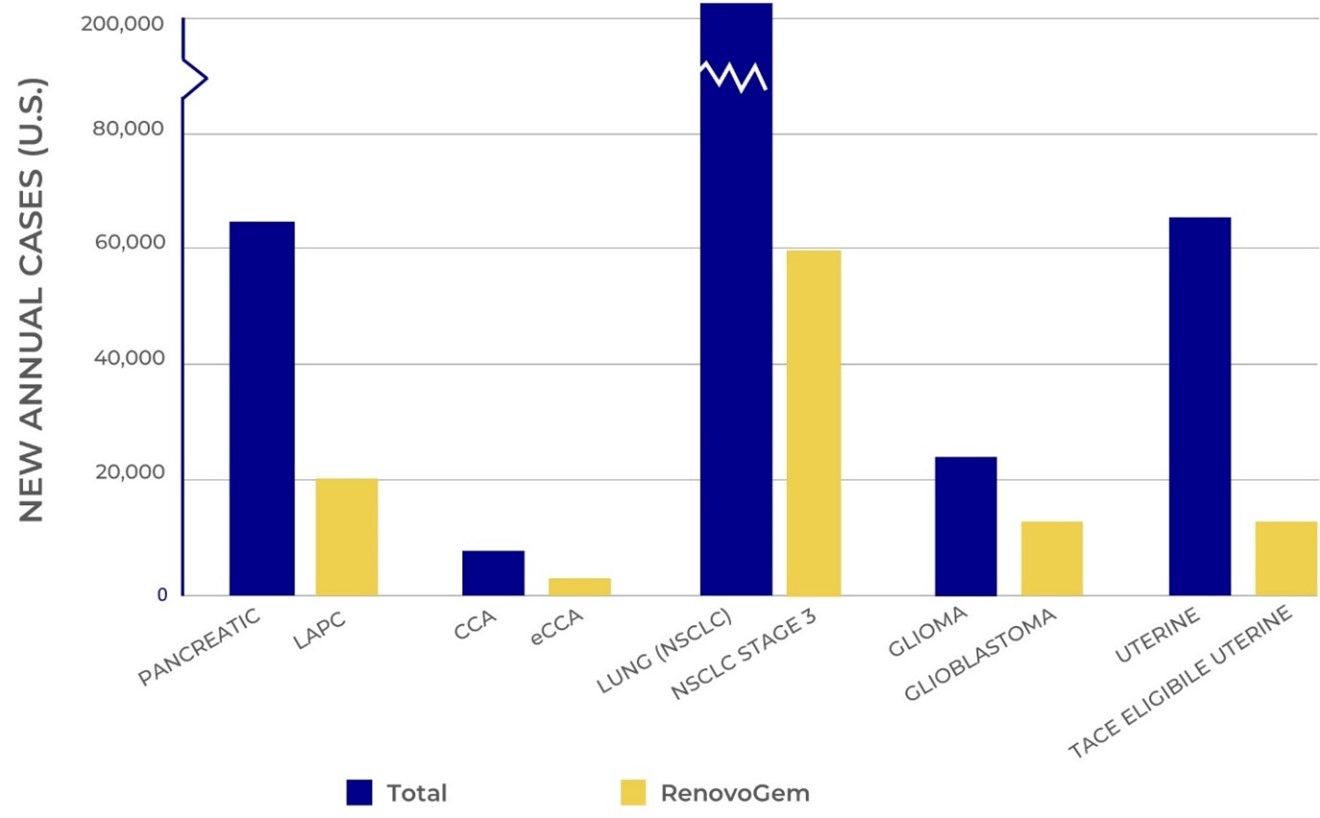
Figure 21: US Annual Incidence of Initial RenovoGem Target Tumor Types, showing overall incidence in blue, and those treatable via RenovoGem in yellow.
| 28 |
| ● | All Pancreatic Cancers compared to Locally Advanced Pancreatic Cancers (LAPC) | |
| ● | All Cholangiocarcinoma (CCA) compared to Extrahepatic CCA | |
| ● | All Non-Small Cell Lung Cancers (NSCLC) compared to Stage 3 NSCLC | |
| ● | All Glioma compared to Glioblastoma | |
| ● | All Uterine cancers versus Transcatheter arterial chemoembolization (TACE) eligible uterine cancers |
We believe TAMP is broadly applicable to locally advanced tumors: our platform may be used with multiple small molecule chemotherapeutic agents in multiple solid tumor indications.
Potential for Separate Commercialization of RenovoCath
Our primary business is to progress RenovoGem through clinical trials for LPAC and other indications with the goal of receiving FDA approval to market these product candidates. In addition, we may explore a strategy where, depending on a number of factors, we may consider selling RenovoCath as a standalone catheter (i.e., without any specific cancer therapeutic) for use by oncology surgeons. We expect to research this opportunity during 2024 by exploring the potential addressable market, regulatory requirements, sales and marketing strategies and the potential for insurance reimbursement. No assurances can be given that we will ultimately pursue this opportunity or, if we do, that we will be able achieve all of the prerequisites necessary for such commercialization or to generate any meaningful revenues from such commercialization.
Intellectual Property
Our success depends in part on our ability to obtain patents and trademarks, maintain trade secret and know-how protection, enforce our proprietary rights against infringers, and operate without infringing on the proprietary rights of third parties. Because of the length of time and expense associated with developing new products and bringing them through the regulatory approval process, the health care industry places considerable emphasis on obtaining patent protection and maintaining trade secret protection for new technologies, products, processes, know-how, and methods.
Our intellectual property protection stems from several issued device and method patents on our RenovoCath delivery system that optimizes delivery of the anti-cancer drug and the TAMP therapy platform. Our issued patents also provide exclusivity as it relates to utilizing RenovoCath with anti-cancer drugs.
We have 8 US patents issued, 1 European patent issued, and 8 additional patents pending in the US, EU, and Asia. We continue to explore additional opportunities to further bolster our IP position. The table below describes our issued patents, all of which have been assigned to us.
| 29 |
| Family | App. No. Filing Date | Type of Patent Protection | Patent Focus | Patent # | Estimated Expiration** | |||||
| Dual Balloon Methods and Apparatuses | 12/958711 Filed: 12/2/2010 | US Utility patent | Methods: isolating splenic artery with 2 balloons (sliding inner catheter) | US8,821,476 | January 25, 2033 | |||||
| Dual Balloon Methods and Apparatuses | 14/870833 Filed: 9/30/2015 | US Utility patent | Apparatus: 2 balloons, seal to isolate lumen, and infusion aperture | US9,463,304 | December 2, 2030 | |||||
| Dual Balloon Methods and Apparatuses | 14/293603 Filed: 6/2/2014 | US Utility patent | Apparatus: 2 balloons (sliding inner catheter), 2 ports for fluid handling | US9,457,171 | April 16, 2031 | |||||
| Dual Balloon Methods and Apparatuses | 15/351922 Filed: 11/15/2016 | US Utility patent | Kits for chemotherapy including catheter with 2 balloons, an infusion aperture and 2 ports | US10,512,761 | April 16, 2031 | |||||
| Dual Balloon Methods and Apparatuses | 108351107 Filed: 12/2/2010 | E.U. Utility patent, nationalized in BE, CH, DE, ES, FR, GB, IE, IT and NL | A two occlusion element, adjustable delivery apparatus having inner and outer catheter, seal to isolate lumen | EU 2506913 | December 2, 2030 | |||||
| Side Branch Isolation Device and Methods | 14/958428 Filed: 12/3/2015 | US Utility patent | Apparatuses and Methods: 3 balloon catheters for isolating side branches | US10,099,040 | December 3, 2035 | |||||
| Trans-Arterial Micro-Perfusion (TAMP) | 15/807011 Filed: 11/8/2017 | US Utility patent | Methods delivering radiation to devascularize then TAMP | U.S., 10,695,543 | August 28, 2038 | |||||
| Dual Balloon Methods and Apparatuses | 18/149649 Filed: 1/3/2023 | US Utility patent (Pending application) | Methods of treating bile duct | PENDING | December 2, 2030 | |||||
| Trans-Arterial Micro-Perfusion (TAMP) | 16/685974 Filed: 11/15/2019 | US Utility patent (Pending application) | Devascularization in conjunction with TAMP | US11,052,224 | November 8, 2037 | |||||
| Dual Balloon Methods and Apparatuses | IN 1632MUMNP2012 Filed: 12/2/2010 | IN Utility patent (Pending application) | A two occlusion element, adjustable delivery apparatus having inner and outer catheter, seal to isolate lumen | PENDING | December 2, 2030 | |||||
| Trans-Arterial Micro-Perfusion (TAMP) | CN 2018800033529 Filed: 5/18/2018 | CN Utility patent (Pending application) | Devascularization in conjunction with TAMP | PENDING | November 8, 2037* | |||||
| Trans-Arterial Micro-Perfusion (TAMP) | EP 187315908 Filed: 5/18/2018 | EP Utility patent (Pending application) | Devascularization in conjunction with TAMP | PENDING | November 8, 2037* | |||||
| Trans-Arterial Micro-Perfusion (TAMP) | JP 2020514151 Filed: 5/18/2018 | JP Utility patent (Pending application) | Devascularization in conjunction with TAMP | PENDING | November 8, 2037* | |||||
| Trans-Arterial Micro-Perfusion (TAMP) | 17/315220 Filed: 5/7/2021 | US Utility patent (Pending application) | Devascularization in conjunction with TAMP | PENDING | November 8, 2037* | |||||
| Trans-Arterial Micro-Perfusion (TAMP) | 17/367046 Filed: 7/2/21 | US Utility patent (Pending application) | Devascularization in conjunction with TAMP | PENDING | November 8, 2037* | |||||
| Dual Balloon Methods and Apparatuses | 17/558577 Filed: 12/21/2021 | US Utility patent (Pending application) | Methods of treating bile duct | US11,541,211 | December 2, 2030 | |||||
| Improved Dual Balloon Methods and Apparatuses | 18/184620 Filed: 3/15/2023 | US Utility patent (Pending application) | Dual occlusion catheter systems | PENDING | March 15, 2043* | |||||
| Microvascular Delivery of Materials | 63/512903 Filed: 07/10/2023 | US Provisional patent (Pending application) | Methods and apparatuses for delivering an agent through Vasa Vasorum | PENDING | N/A | |||||
| Microvascular Delivery of Materials | PCT/US2023/037221 Filed: 11/13/2023 | International PCT application (Pending application) | Methods and devices for delivering agent through Vasa Vasorum | PENDING | N/A |
* Predicted earliest expiration date. The actual expiration date will depend on factors related to patent prosecution and issuance.
** Estimated expiration dates assume all maintenance fees are paid.
| 30 |
Orphan drug designation provides seven years post-approval market exclusivity protection. Gemcitabine is generic; however, we have exclusivity for the IA route of administration. RenovoGem is regulated by the FDA as a new oncology drug product. We intend to make IA gemcitabine and RenovoCath available as a combined product and not to make either component available separately. Once approved, we will have exclusivity over the use of IA gemcitabine as it will be approved by the FDA in combination with RenovoCath.
When appropriate, we actively pursue protection of our proprietary products, technologies, processes, and methods by filing United States and international patent and trademark applications. We seek to pursue additional patent protection for technology invented through research and development, manufacturing, and clinical use of our technology that will enable us to expand our patent portfolio around advances to our current systems, technology, and methods for our current applications as well as others.
There can be no assurance that the pending patent applications will result in the issuance of patents, that patents issued to or licensed by us will not be challenged or circumvented by competitors, or that these patents will be found to be valid or sufficiently broad to protect our technology or provide us with a competitive advantage.
To maintain our proprietary position, we also rely on trade secrets and proprietary technological experience to protect proprietary manufacturing processes, technology, and know-how relating to our business. We rely, in part, on confidentiality agreements with our marketing partners, employees, advisors, vendors and consultants to protect our trade secrets and proprietary technological expertise. In addition, we also seek to maintain our trade secrets through maintenance of the physical security of the premises where our trade secrets are located. There can be no assurance that these agreements will not be breached, that we will have adequate remedies for any breach, that others will not independently develop equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets and proprietary knowledge.
In certain circumstances, United States patent law allows for the extension of a patent’s duration for a period of up to five years after FDA approval. We intend to seek extension for one of our patents after FDA approval if it has not expired prior to the date of approval. In addition to our proprietary protections, the FDA has granted us two orphan drug designations that provide us a seven-year period of exclusive marketing beginning on the date that our NDA is approved by the FDA for the designated orphan drug. While exclusivity only applies to the indication for which the drug has been approved, we believe that this exclusivity will provide us with added protection once commercialization of an orphan drug designated product begins.
There has been and continues to be substantial litigation regarding patent and other intellectual property rights in the pharmaceutical and medical device areas. If a third party asserts a claim against us, we may be forced to expend significant time and money defending such actions and an adverse determination in any patent litigation could subject us to significant liabilities to third parties, require us to redesign our product, require us to seek licenses from third parties, and, if licenses are not available, prevent us from manufacturing, selling or using our system. Additionally, we plan to enforce our intellectual property rights vigorously and may find it necessary to initiate litigation to enforce our patent rights or to protect our trade secrets or know-how. Patent litigation can be costly and time consuming and there can be no assurance that the outcome will be favorable to us.
| 31 |
Manufacturing and Supply
For the catheter component (RenovoCath) of our drug/device combination, we currently rely on a single-source contract manufacturer, Medical Murray, North Barrington, IL. However, we are in early discussions with an additional manufacturer. We are subject to regulatory requirements of the FDA’s Quality System Regulation (QSR), for medical devices sold in the United States, and the European Medical Device Directive 93/42/EEC, which was replaced by the EU Medical Device Regulation (MDR) in May 2021, following a four-year transition period for medical devices marketed in the European Union. We have an agreement in place with Medical Murray to produce the RenovoCath through October 2024 with automatic annual renewal until termination by either party with 12 months’ notice. While we believe Medical Murray has the capabilities to scale RenovoCath production to peak forecasted commercial volumes, manufacturing can be transferred to additional vendors if needed.
The FDA monitors compliance with QSR through periodic inspections of both our facility and the facility of our contact manufacturer. Our European Union Notified Body, British Standards Institute (BSI), monitors compliance with the MDR requirements through both annual scheduled audits and periodic unannounced audits of our facilities as well as our contract manufacturer’s facilities.
Our failure or the failure of our contract manufacturer to maintain acceptable quality requirements could result in the shutdown of our manufacturing operations or the recall of products which could be detrimental to our company. If our contract manufacturer fails to maintain acceptable quality requirements, we may have to qualify a new contract manufacturer and could experience a material adverse effect to manufacturing and manufacturing delays as a result.
We do not own or operate and do not intend to establish our own gemcitabine manufacturing facilities.
Within our TIGeR-PaC Phase III trial, hospitals are sourcing generic gemcitabine labeled for IV use from their respective pharmacies to use in conjunction with the RenovoCath for the TAMP procedures. In the commercial setting, we expect to rely on contract manufacturing organizations for gemcitabine production, relabeling and co-packaging with the RenovoCath. The formulation of gemcitabine used in the TIGeR-PaC Phase III trial and in the commercial setting will be identical, however, the labeling of gemcitabine will be IA gemcitabine to be used exclusively in conjunction with RenovoCath.
Government Regulation
Our products are subject to extensive and rigorous government regulation by foreign regulatory agencies and the FDA. Foreign regulatory agencies, the FDA and regulatory agencies in state and local jurisdictions impose extensive requirements upon the clinical development, pre-market clearance and approval, manufacturing, labeling, marketing, advertising and promotion, pricing, storage and distribution of pharmaceutical and medical device products. Failure to comply with applicable foreign regulatory agency or FDA requirements may result in warning letters, fines, civil or criminal penalties, suspension or delays in clinical development, recall or seizure of products, partial or total suspension of production or withdrawal of a product from the market.
United States Regulatory Environment
In the US, the FDA regulates drug and device products, including combination, under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and its implementing regulations. RenovoGem is subject to FDA regulation in 21 CFR 3.2(e) as a combination product, which means it is composed of both a drug component and device component. Each component of a combination product is subject to the requirements established by FDA for that type of component and if marketed individually, each component would be subject to different regulatory pathways and reviewed by different centers within the FDA. A combination product, however, is assigned to a center that will have primary jurisdiction over its pre-market review and regulation based on a determination of its primary mode of action, which is the single mode of action that provides the most important therapeutic action. The center that regulates the portion of the product that has the primary mode of action becomes the lead evaluator. When evaluating an NDA the lead center may consult other centers but still retain complete reviewing authority, or it may collaborate with another center, by which the center assigns review of a specific section of the application to another center, delegating its review authority for that section. Typically, an applicant submits a single marketing application to the center selected to be the lead evaluator, although separate applications for each constituent part may be submitted to the applicable centers. Combination products where the drug provides the primary mechanism of action are often referred to as “drug-led combination” products.
| 32 |
In a drug/device combination product, containing two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, where the primary mode of action is typically a drug mode of action with the Center for Drug Evaluation and Research (“CDER”), as the lead Center, CDER would review the NDA in consultation with the Center for Devices and Radiological Health (“CDRH”) on device-specific issues. For co-packaged or single-entity combination products there are two ways to comply with current good manufacturing practice (“cGMP”) requirements. Manufacturers can either (i) demonstrate compliance with all cGMP regulations applicable to each of the constituent parts in the combination product or (ii) in the case of drug/device combination products, demonstrate compliance with either the drug cGMP regulations or the device Quality System Regulation, or QSR, and also demonstrate compliance with additional provisions from the other of these two sets of cGMP requirements, as specified in the combination products regulations. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions: warning or untitled letters, fines, injunctions, civil or criminal penalties, recall or seizure of current or future products, operating restrictions, partial suspension or total shutdown of production, refusal or denial of submissions for new products, or withdrawal of clearance, authorization, or approval.
In the case of RenovoGem, the primary mode of action is attributable to the drug component of the product, which means that CDER, has primary jurisdiction over its pre-market development and review. The underlying RenovoCath drug delivery device system used in RenovoGem has been separately cleared by CDRH as a Class II medical device as a standalone device and not in combination of a specific drug product or part of a prepackaged combination product for the isolation of blood flow and delivery of fluids, including diagnostic and/or therapeutic agents, to selected sites in the peripheral vascular system. The RenovoCath is also indicated for temporary vessel occlusion in applications including arteriography, preoperative occlusion, and chemotherapeutic drug infusion. The RenovoCath is intended for general intravascular use in the peripheral vasculature in arteries 3mm and larger. The RenovoCath is intended for use in arteries from 3mm in diameter for vessel entry and to occlude vessels ranging between 3mm to 11mm in diameter.
The process required by the FDA before drug product candidates, including drug-led combination products, may be marketed in the United States generally involves the following:
| ● | submission to the FDA of an IND, which must become effective before human clinical trials may begin and must be updated periodically; | |
| ● | completion of extensive preclinical laboratory tests and animal studies, all performed in accordance with the FDA’s Good Laboratory Practice, or GLP, regulations; | |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product candidate for each proposed indication; | |
| ● | submission to the FDA of an NDA after completion of all registrational or pivotal clinical trials; | |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the product is produced and tested to assess compliance with cGMP regulations; and | |
| ● | FDA review and approval of an NDA prior to any commercial marketing or sale of the drug in the United States. |
The development and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product will be granted on a timely basis, if at all.
The results of preclinical tests (which include laboratory evaluation as well as GLP studies to evaluate toxicity in animals) for a particular product candidate, together with related manufacturing information and analytical data, are submitted as part of an IND to the FDA. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises concerns or questions about the conduct of the proposed clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. IND submissions may not result in FDA authorization to commence a clinical trial. A separate submission to an existing IND must also be made for each successive clinical trial conducted during product development. Further, an independent institutional review board (“IRB”), for each medical center proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that center and it must monitor the study until completion. The FDA, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive good clinical practice (“GCP”), regulations and regulations for informed consent and privacy of individually identifiable information. Similar requirements to the United States, Clinical Trial Applications (“CTA”) are required in the EU and other jurisdictions to initiate clinical trials.
| 33 |
Clinical Trials
For purposes of NDA submission and approval, clinical trials are typically conducted in the following sequential phases, which may overlap:
| ● | Phase I Clinical Trials. Studies are initially conducted in a limited population to test the product candidate for safety, dose tolerance, absorption, distribution, metabolism and excretion, typically in healthy humans, but in some cases in patients. | |
| ● | Phase II Clinical Trials. Studies are generally conducted in a limited patient population to identify possible adverse effects and safety risks, explore the initial efficacy of the product for specific targeted indications and to determine dose range or pharmacodynamics. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more expensive Phase III clinical trials. | |
| ● | Phase III Clinical Trials. These are commonly referred to as pivotal studies. When Phase II evaluations demonstrate that a dose range of the product is effective and has an acceptable safety profile, Phase III clinical trials are undertaken in large patient populations to further evaluate dosage, provide substantial evidence of clinical efficacy and further test for safety in an expanded and diverse patient population at multiple, geographically dispersed clinical trial centers. | |
| ● | Phase IV Clinical Trials. The FDA may approve an NDA for a product candidate, but require that the sponsor conduct additional clinical trials to further assess the drug after NDA approval under a post-approval commitment. In addition, a sponsor may decide to conduct additional clinical trials after the FDA has approved an NDA. Post-approval trials are typically referred to as Phase IV clinical trials. |
Since the COVID-19 public health emergency, FDA has issued various COVID-19 guidance documents, including guidance on conducting clinical trials during the pandemic; resuming normal drug and biologics manufacturing operations; manufacturing, supply chain, and inspections; and statistical considerations for clinical trials during the COVID-19 public health emergency, among others. The impact of the COVID-19 pandemic on business like ours has begun to recede. However, if new pandemic diseases occur, or if new regulatory requirements and changes to existing regulations. If new guidance and policies are promulgated by the FDA that require changes in our clinical protocol or clinical development plans, our anticipated timelines and regulatory approval may be delayed or materially impacted. President Biden ended the COVID-19 national and public health emergencies on May 11, 2023. However, the full impact of the termination of the public health emergencies on FDA and other regulatory policies and operations remain unclear.
| 34 |
New Drug Applications (NDAs)
The results of drug development, preclinical studies and clinical trials are submitted to the FDA as part of an NDA. NDAs also must contain extensive chemistry, manufacturing and control information. An NDA must be accompanied by a significant user fee, which may be waived in certain circumstances. Once the submission has been accepted for filing, the FDA’s goal is to review applications within ten months of submission or, if the application relates to an unmet medical need in a serious or life-threatening indication, six months from submission. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. For new oncology products, the FDA will often solicit an opinion from an Oncologic Drugs Advisory Committee, or ODAC, a panel of expert authorities knowledgeable in the fields of general oncology, pediatric oncology, hematologic oncology, immunologic oncology, biostatistics, and other related professions. The ODAC panel reviews and evaluates data concerning the safety and effectiveness of marketed and investigational human drug products for use in the treatment of cancer, and makes appropriate recommendations to the Commissioner of Food and Drugs. The FDA is not bound by the recommendation of an advisory committee.
Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will generally inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with cGMPs is satisfactory, and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied. After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter (“CRL”). A CRL generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. A CRL may require additional clinical data and/or an additional pivotal Phase III clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we or our collaborators interpret data. Approval may be contingent on a Risk Evaluation and Mitigation Strategy (“REMS”) that limits the labeling, distribution or promotion of a drug product. Once issued, the FDA may withdraw product approval if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing, including Phase IV clinical trials, and surveillance programs to monitor the safety effects of approved products which have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs or other information.
There are three primary regulatory pathways for an NDA under Section 505 of the FDCA: Section 505 (b)(1), Section 505 (b)(2) and Section 505(j). A Section 505 (b)(1) application is used for approval of a new drug (for clinical use) whose active ingredients have not been previously approved. A Section 505 (b)(2) application is used for a new drug that relies on data not developed by the applicant. Section 505(b)(2) of the FDCA was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act. This statutory provision permits the approval of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. The Hatch-Waxman Act permits the applicant to rely in part upon the FDA’s findings of safety and effectiveness for previously approved products. Section 505(j) application, also known as an abbreviated NDA, is used for a generic version of a drug that has already been approved.
An approval letter authorizes commercial marketing of the drug product with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a REMS to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
| 35 |
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
The NDA review process for drug-led combination includes a review of the device constituent. In this case, the device constituent for RenovoGem is RenovoCath, which is cleared by the FDA.
Orphan Drug Exclusivity
Some jurisdictions, including the United States, may designate drugs for relatively small patient populations as orphan drugs. Pursuant to the Orphan Drug Act, the FDA grants orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States. The orphan designation is granted for a combination of a drug entity and an indication and therefore it can be granted for an existing drug with a new (orphan) indication. Applications are made to the Office of Orphan Products Development at the FDA and a decision or request for more information is rendered in 60 days. NDAs for designated orphan drugs are exempt from user fees, obtain additional clinical protocol assistance, are eligible for tax credits up to 50% of research and development costs, and are granted a seven-year period of exclusivity upon approval. The FDA cannot approve the same drug for the same condition during this period of exclusivity, except in certain circumstances where a new product demonstrates superiority to the original treatment. Exclusivity begins on the date that the marketing application is approved by the FDA for the designated orphan drug, and an orphan designation does not limit the use of that drug in other applications outside the approved designation in either a commercial or investigational setting.
We have received orphan drug designations for RenovoGem for pancreatic cancer and cholangiocarcinoma.
The granting of orphan drug designations does not mean that the FDA has approved a new drug. Companies must still pursue the rigorous development and approval process that requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product will be granted at all, or on a timely basis.
FDA Medical Device Regulation
Unless an exemption applies, each new or significantly modified drug delivery medical device that we develop based on the current 510(k)-cleared RenovoCath and which we seek to commercially distribute in the United States will require a premarket notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, unless addressed as part of a new drug application for a drug/device combination product. New medical devices without an applicable predicate device as well as higher-risk medical devices are subject to premarket approval by the FDA under a PMA or a de novo classification from the FDA. The 510(k) clearance, PMA approval, and de novo classification processes can be resource intensive, expensive, and lengthy, and require payment of significant user fees, unless an exemption is available.
Under the FDCA, medical devices are classified into one of three classes – Class I, Class II or Class III – depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to ensure its safety and effectiveness.
| 36 |
Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be reasonably assured by adherence to a set of FDA regulations, referred to as the general controls for medical devices, which require compliance with the applicable portions of cGMP regulations known as the Quality System Regulation, or QSR, facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful, and non-misleading labeling and promotional materials. Some Class I devices, also called Class I reserved devices, also require premarket clearance by the FDA through the 510(k) premarket notification process described below. Most Class I products are exempt from the premarket notification requirements.
Class II devices are those that are subject to the general controls and special controls, as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, patient registries, FDA guidance documents, and post-market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process.
Class III devices include devices deemed by the FDA to pose the greatest risk such as life-supporting or life-sustaining devices, or implantable devices, or devices that have a new intended use or use advanced technology that are not substantially equivalent to that of a legally marketed predicate device. The safety and effectiveness of Class III devices cannot be reasonably assured solely by the general controls and special controls described above. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption (IDE), regulations which govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. If the device under evaluation does not present a significant risk to human health, then the device sponsor is not required to submit an IDE application to the FDA before initiating human clinical trials, but must still comply with abbreviated IDE requirements when conducting such trials. Therefore, these devices are subject to the PMA process, which is generally more costly and time consuming than the 510(k) process.
The 510(k) Clearance Process
Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent,” as defined in the statute, to a legally marketed predicate device. A predicate device is a legally marketed device that is not subject to premarket approval, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I, or a device that was previously found substantially equivalent through the 510(k) process. The FDA’s 510(k) clearance process usually takes from three to 12 months, but may take longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence. In addition, FDA collects user fees for certain medical device submissions and annual fees and for medical device establishments.
If the FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant 510(k) clearance to commercially market the device. If the FDA determines that the device is not “substantially equivalent” to a predicate device, the device is automatically classified into Class III. The device sponsor must then fulfill the much more rigorous premarketing requirements of the PMA approval process, or seek risk-based reclassification of the device through the de novo process, which is a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device. A manufacturer can also submit a petition for direct de novo review if the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk.
After a device receives 510(k) clearance or de novo classification, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA or de novo classification. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k) or a PMA in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. Many minor modifications are accomplished by a letter-to-file in which the manufacturer documents the change in an internal letter-to-file. The letter-to-file is in lieu of submitting a new 510(k) to obtain clearance for such change. The FDA can always review these letters to file in an inspection. If the FDA disagrees with a manufacturer’s determination regarding whether a new premarket submission is required for the modification of an existing device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until marketing authorization is obtained. Also, in these circumstances, the manufacturer may be subject to significant regulatory fines or penalties.
| 37 |
Other FDA Regulatory Requirements
Products manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including recordkeeping, annual product quality review and reporting requirements. Adverse event experiences with the product, including both drug-related and device-related adverse events (including device malfunctions and medical device reporting), must be reported to the FDA in a timely fashion and pharmacovigilance programs to proactively look for these adverse events are mandated by the FDA. Drug and device manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP and QSR, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Following such inspections, the FDA may issue notices on Form 483, Untitled Letters or Warning Letters that could cause us or our third-party manufacturers to modify certain activities. A Form 483 Notice, if issued at the conclusion of an FDA inspection, list conditions the FDA investigators believe may have violated cGMP, QSR or other FDA regulations or guidelines. In addition to Form 483 Notices and Untitled Letters or Warning Letters, failure to comply with the statutory and regulatory requirements can subject a manufacturer to possible legal or regulatory action, such as suspension of manufacturing, seizure of product, injunctive action or possible civil penalties. We cannot be certain that we or our present or future third-party manufacturers or suppliers will be able to comply with the cGMP regulations, QSR and other ongoing FDA regulatory requirements. If we or our present or future third-party manufacturers or suppliers are not able to comply with these requirements, the FDA may require us to recall our products from distribution or withdraw any potential approvals of an NDA for that product.
The FDA closely regulates the post-approval marketing and promotion of drugs and devices, including standards and regulations for direct-to-consumer advertising, dissemination of off-label information, industry-sponsored scientific and educational activities and promotional activities involving the Internet. Drugs and devices may be marketed only for the approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to the drug or device, including changes in indications, labeling, or manufacturing processes or facilities, we may be required to submit and obtain FDA approval of a new or supplemental NDA or clearance or approval of the modified device, respectively, which may require us to develop additional data or conduct additional preclinical studies and clinical trials. Failure to comply with these requirements can result in adverse publicity, Warning Letters, corrective advertising and potential civil and criminal penalties.
Physicians may prescribe legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties, in particular in oncology. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, impose stringent restrictions on manufacturers’ communications regarding off-label use.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act (“PDMA”), which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Foreign Regulatory Environment
If we seek to market RenovoGem in foreign jurisdictions, we could become subject to a variety of foreign regulations regarding development, approval, commercial sales, and distribution of our products in addition to regulations in the United States. Whether or not we obtain FDA approval for a product, we must obtain the necessary approvals by the regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and can involve additional product testing and additional review periods. The review process may take longer or shorter than that required to obtain FDA approval. The requirements governing, among other things, the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. If we fail to comply with applicable foreign regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, and/or criminal prosecution.
| 38 |
Other United States Healthcare Laws
In addition to FDA restrictions on marketing of pharmaceutical and medical device products, including drug/device combination products, pharmaceutical companies are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which they conduct their business and may constrain the financial arrangements and relationships through which we research, as well as sell, market and distribute any products for which we obtain marketing authorization. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, data privacy and security, and transparency laws and regulations related to drug pricing and payments and other transfers of value made to physicians and other healthcare providers. If manufacturers’ operations, including activities engaged by their contractors or agents, are found to be in violation of any of such laws or any other governmental regulations that apply, they may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of operations, integrity oversight and reporting obligations, exclusion from participation in federal and state healthcare programs and imprisonment.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. The Affordable Care Act, or ACA, amended the intent element of the federal statute so that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to commit a violation. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers, among others, on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting certain business activities from prosecution or other regulatory sanctions, the exceptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor.
Federal civil and criminal false claims laws, including the federal civil False Claims Act, prohibit any person or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. This includes claims made to programs where the federal government reimburses, such as Medicare and Medicaid, as well as programs where the federal government is a direct purchaser, such as when it purchases off the Federal Supply Schedule. Pharmaceutical and other healthcare companies have been prosecuted under these laws for, among other things, allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. Additionally, the ACA amended the federal Anti-Kickback Statute such that a violation of that statute can serve as a basis for liability under the federal False Claims Act. Most states also have statutes or regulations similar to the federal Anti-Kickback Statute and civil False Claims Act, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Other federal statutes pertaining to healthcare fraud and abuse include the civil monetary penalties statute, which prohibits, among other things, the offer or payment of remuneration to a Medicaid or Medicare beneficiary that the offeror or payor knows or should know is likely to influence the beneficiary to order a receive a reimbursable item or service from a particular supplier, and the additional federal criminal statutes created by the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits, among other things, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program or obtain by means of false or fraudulent pretenses, representations or promises any money or property owned by or under the control of any healthcare benefit program in connection with the delivery of or payment for healthcare benefits, items or services.
| 39 |
In addition, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and their respective implementing regulations, impose obligations on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their business associates that perform certain services involving the storage, use or disclosure of individually identifiable health information, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information, and require notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information. HITECH increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, many state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, and often are not preempted by HIPAA.
Further, pursuant to the ACA, the Centers for Medicare and Medicaid Services (“CMS”) has issued a final rule that requires applicable manufacturers of covered products, including prescription drugs and certain medical devices, to collect and annually report information on certain payments or transfers of value made in the previous year to physicians (defined to include doctors of medicine and osteopathy, dentists, podiatrists, optometrists and licensed chiropractors), certain non-physician healthcare professionals (such as physician assistants and nurse practitioners, among others) and teaching hospitals, as well information regarding investment interests held by physicians and their immediate family members. The reported data is made available in searchable form on a public website on an annual basis. Failure to submit required information may result in civil monetary penalties. In addition, several states now require prescription drug companies to report certain expenses relating to the marketing and promotion of drug products and to report gifts and payments to individual healthcare practitioners in these states. Other states prohibit various marketing-related activities and/or require the posting of information relating to clinical studies and their outcomes. Some states require the reporting of certain pricing information, including information pertaining to and justifying price increases, or prohibit prescription drug price gouging. In addition, certain states require pharmaceutical companies to implement a healthcare compliance program or code of conduct. Certain states and local jurisdictions also require the registration of pharmaceutical sales representatives. Compliance with these federal and state laws is difficult and time consuming, and companies that do not comply with these state laws are exposed to liabilities and civil penalties.
Efforts to ensure that business arrangements with third parties comply with applicable healthcare laws and regulations involve substantial costs. If a drug company’s operations are found to be in violation of any such requirements, it may be subject to significant penalties, including civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, the curtailment or restructuring of its operations, loss of eligibility to obtain approvals from the FDA, exclusion from participation in government contracting, healthcare reimbursement or other federal or state government healthcare programs, including Medicare and Medicaid, integrity oversight and reporting obligations, imprisonment, and reputational harm. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action for an alleged or suspected violation can cause a drug company to incur significant legal expenses and divert management’s attention from the operation of the business, even if such action is successfully defended.
Healthcare Reform
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, each as amended, collectively known as the ACA, was enacted, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly affected the pharmaceutical industry. The ACA contained a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement adjustments and changes to fraud and abuse laws. Additionally, the ACA:
| ● | increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1% of the average manufacturer price; | |
| ● | required collection of rebates for drugs paid by Medicaid managed care organizations; |
| 40 |
| ● | required manufacturers to participate in a coverage gap discount program, under which they must agree to offer 70 percent point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and | |
| ● | imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specified federal government programs. |
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. For example, in June 2021 the U.S. Supreme Court held that Texas and other challengers had no legal standing to challenge the ACA, dismissing the case on procedural grounds without specifically ruling on the constitutionality of the ACA. Thus, the ACA will remain in effect in its current form. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and healthcare measures promulgated by the Biden administration will impact the ACA, our business, financial condition and results of operations.
Other legislative changes have been proposed and adopted since the ACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2031, with the exception of a temporary suspension implemented under various COVID-19 relief legislation from May 1, 2020 through March 31, 2022, unless additional action is taken by Congress. Under current legislation, the actual reduction in Medicare payments can vary from 1% in 2022 to up to 4% in the final fiscal year of this sequester.
Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. For example, under the American Rescue Plan Act of 2021, effective January 1, 2024, the statutory cap on Medicaid Drug Rebate Program rebates that manufacturers pay to state Medicaid programs will be eliminated. Elimination of this cap may require pharmaceutical manufacturers to pay more in rebates than it receives on the sale of products, which could have a material impact on our business. In August 2022, Congress passed the Inflation Reduction Act of 2022, which includes prescription drug provisions that have significant implications for the pharmaceutical industry and Medicare beneficiaries, including allowing the federal government to negotiate a maximum fair price for certain high-priced single source Medicare drugs, imposing penalties and excise tax for manufacturers that fail to comply with the drug price negotiation requirements, requiring inflation rebates for all Medicare Part B and Part D drugs, with limited exceptions, if their drug prices increase faster than inflation, and redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, among other changes. On June 30, 2023, CMS issued new guidance detailing the requirements and parameters of the first round of price negotiations, to take place during 2023 and 2024, for products subject to the “maximum fair price” provision that would become effective in 2026. On August 29, 2023, the U.S. Department of Health and Human Services (“HHS”) announced the list of the first ten drugs that will be subject to price negotiations, although the Medicare drug price negotiation program is currently subject to legal challenges. CMS and HHS will continue to issue and update guidance as these programs are implemented. The impact of these regulations and any future healthcare measures and agency rules implemented by the Biden administration on us and the pharmaceutical industry as a whole is currently unknown.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. For example, a number of states are considering or have recently enacted state drug price transparency and reporting laws that could substantially increase our compliance burdens and expose us to greater liability under such state laws once we begin commercialization after obtaining regulatory approval for any of our products.
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidate for which we may seek regulatory approval. Sales in the U.S. will depend, in part, on the availability of sufficient coverage and adequate reimbursement from third-party payors, which include government health programs such as Medicare, Medicaid, TRICARE and the Veterans Administration, as well as managed care organizations and private health insurers. Prices at which we or our customers seek reimbursement for our product candidates can be subject to challenge, reduction or denial by third-party payors.
| 41 |
The process for determining whether a third-party payor will provide coverage for a product is typically separate from the process for setting the reimbursement rate that the payor will pay for the product. A third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be available. Additionally, in the U.S. there is no uniform policy among payors for coverage or reimbursement. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies, but also have their own methods and approval processes. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. If coverage and adequate reimbursement are not available, or are available only at limited levels, successful commercialization of, and obtaining a satisfactory financial return on, any product we develop may not be possible.
Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In order to obtain coverage and reimbursement for any product that might be approved for marketing, we may need to conduct expensive studies in order to demonstrate the medical necessity and cost-effectiveness of any products, which would be in addition to the costs expended to obtain regulatory approvals. Third-party payors may not consider our product candidates to be medically necessary or cost-effective compared to other available therapies, or the rebate percentages required to secure favorable coverage may not yield an adequate margin over cost or may not enable us to maintain price levels sufficient to realize an appropriate return on our investment in drug development.
We are developing a new drug product, RenovoGem, which is IA gemcitabine delivered via the proprietary RenovoCath delivery system. If the drug is approved, it is expected to be sold together with the catheter used to administer the drug in the National Drug Code (NDC) created when the drug receives FDA approval. The reimbursement pathway involves separate payments for the drug product and for the occlusion procedure to administer it. As to the latter, it is anticipated that the procedure is accurately described by an existing code with existing payment levels. Given the expectation that the drug will be a novel, non-generic drug, a unique code and payment based on pricing information for the product should be established.
For the reasons discussed above, we believe there is a clear path to reimbursement for RenovoGem and its related procedure in both the hospital outpatient and physician office settings (which may include freestanding entities such as catheterization laboratories). As is typical for a product still in clinical development, it is difficult to predict whether there would be any Medicare coverage obstacles, which there usually are not for FDA approved drugs being used for on-label use. We believe the most important step we can take to enhance reimbursement for our products is the development of published, peer-reviewed clinical literature supporting their clinical benefit. While we may also explore commercial opportunities to sell RenovoCath as a standalone catheter, which would require its own insurance reimbursement arrangements.
Competition
The oncology biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies and strong competition. While we believe that our knowledge, leadership, experience, scientific resources, intellectual property, regulatory barriers, and the advanced stage of our clinical development provide us with competitive advantages, we may face competition from major pharmaceutical companies, specialty pharmaceutical companies, and biotechnology companies, worldwide. Many potential competitors have substantially greater scientific, research, financial, technical, and/or human resources than we do.
Many companies are active in the oncology market both in terms of commercially marketed products and products in development that could potentially compete with our products and product candidates for the treatment of solid tumors. Any product candidates that we successfully develop and commercialize may compete directly with approved and/or new therapies that may be approved in the future. Our competitors may also obtain FDA or foreign regulatory approval for their products more rapidly than we may obtain approval for our product candidates which could result in our competitors establishing a strong market position prior to us entering the market. Key competitive factors affecting the success of our product candidates, if approved, are likely to be their safety, efficacy, convenience, price, and the availability of reimbursement from government and other third-party payors. Many companies are developing new therapeutics and we cannot predict what the standard of care will be as our product candidate progresses through clinical development.
| 42 |
Currently, there are a number of companies in Phase III clinical trials for the treatment of LAPC including AB Science, Angiodynamics, Bausch Health, Fibrogen, NovoCure, SynCore Biotechnology, BMS, and ViewRay. We are aware of a number of companies in Phase I and Phase II clinical trials for the treatment of LAPC including one interventional company, TriSalus Lifesciences.
Many of our competitors have substantially greater financial, technological, research and development, marketing and personnel resources. In addition, some of our competitors have considerable experience in conducting clinical trials, regulatory, manufacturing and commercialization capabilities. Our competitors may develop alternative treatment methods, or achieve earlier product development, in which case the likelihood of us achieving meaningful revenues or profitability will be substantially reduced.
Employees and Human Capital Resources
As of the date of this Report, we have eight employees. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We focus on employee engagement and believe our relationship with our employees is good. We have 16 key consultants in the areas of quality, regulatory, finance, legal, clinical, and marketing.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, and incentivizing our existing employees. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Facilities
Our administrative headquarters is located at 4546 El Camino Real, Suite B1, Los Altos, CA 94022. The office space is approximately 1,600 square feet, and we rent on a month-to-month basis. We believe that our facility is adequate for our current operations and purposes, and that suitable additional or alternative space will be available in the future on commercially reasonable terms, if required.
Legal Proceedings
From time to time, we are engaged in various legal actions, claims and proceedings arising in the ordinary course of business, none of which are expected to be material.
Item 1A. Risk Factors
An investment in our securities is speculative and involves a high degree of risk. You should carefully consider the risks described below, as well as the other information in this Report, including our financial statements and the related notes and the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and in our other public filings in evaluating our business. The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations, growth prospects or stock price. In such an event, the market price of our common stock could decline, and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations and the market price of our common stock.
Risk Factors Summary
The following is a summary of principal factors and uncertainties that make investing in shares of our common stock risky and impact our ability to execute on our business strategy include risks regarding the following. This summary is not exhaustive, and readers are therefore encouraged to review this Risk Factors section in its entirety.
| ● | We are a clinical stage biopharmaceutical company, have a limited operating history and have no drug/device combination products approved for commercial sale, which makes it difficult to evaluate our current business and predict our future success and viability. |
| 43 |
| ● | We have incurred significant net losses in each period since inception, and we expect to continue to incur net losses for the foreseeable future. | |
| ● | We will need to raise substantial additional capital to develop and commercialize RenovoGem, and our failure to obtain funding when needed may force us to delay, reduce or eliminate our product development programs or collaboration efforts. As a result, there is substantial doubt about our ability to operate as a going concern and we estimate that our current capital resources will be sufficient to fund our operating expenses and capital expenditure requirements through the fourth quarter of 2024. | |
| ● | We may consider strategic alternatives in order to maximize stockholder value, including financing, strategic alliances, and licensing arrangements. We may not be able to identify or consummate any suitable strategic alternatives and any consummated strategic alternatives may not be successful. | |
| ● | Our product candidates’ commercial viability remains subject to current and future preclinical studies, clinical trials, regulatory approvals, and the risks generally inherent in the development of a pharmaceutical product candidate. If we are unable to successfully advance or develop our product candidates, our business will be materially harmed. | |
| ● | If we do not achieve our projected development goals in the timeframes we announce and expect, our stock price may decline. | |
| ● | Our product candidates may exhibit undesirable side effects when used alone or in combination with other approved pharmaceutical products or investigational new drugs, which may delay or preclude further development or regulatory approval or limit their use if approved. | |
| ● | If the results of preclinical studies or clinical trials for our product candidates are negative, we could be delayed or precluded from the further development or commercialization of our product candidates, which could materially harm our business. | |
| ● | If we are unable to satisfy regulatory requirements, we may not be able to commercialize our product candidates. | |
| ● | If our product candidates are unable to compete effectively with marketed drugs targeting similar indications as our product candidates, our commercial opportunity will be reduced or eliminated. | |
| ● | We may delay or terminate the development of our product candidates at any time if we believe the perceived market or commercial opportunity does not justify further investment, which could materially harm our business. | |
| ● | Our future success depends on our ability to retain our key personnel and to attract, retain, and motivate qualified personnel, especially in light of an acute workforce shortage and hyper-competitive compensation environment. | |
| ● | If we are unable to protect our intellectual property effectively, we may be unable to prevent third parties from using our technologies, which would impair our competitive advantage. | |
| ● | The patents issued to us may not be broad enough to provide any meaningful protection, one or more of our competitors may develop more effective technologies, designs, or methods without infringing our intellectual property rights and one or more of our competitors may design around our proprietary technologies. | |
| ● | The market price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for our investors.
| |
| ● | If we fail to regain compliance with the applicable Nasdaq continued listing requirement, our common stock may be delisted from Nasdaq. |
| 44 |
Risks Related to Our Business, Financial Condition and Capital Requirements
We are a clinical stage biopharmaceutical company, have a limited operating history and have no drug/device combination products approved for commercial sale, which makes it difficult to evaluate our current business and predict our future success and viability.
We are a clinical stage biopharmaceutical company with a limited operating history upon which you can evaluate our business and prospects. While RenovoCath, a drug delivery medical device, has been separately cleared by the FDA for the isolation of blood flow and delivery of fluids, including diagnostic and/or therapeutic agents, to selected sites in the peripheral vascular system, and for temporary vessel occlusion in applications including arteriography, preoperative occlusion, and chemotherapeutic drug infusion, we are focused on developing and commercializing drug product candidates in combination with our delivery platform technology. We have no drug/device combination products approved for commercial sale and have not generated any revenue from product sales. We are developing a novel therapy platform, which is an unproven and highly uncertain undertaking and involves a substantial degree of risk. Our first product candidate, RenovoGem, is a drug/device combination product consisting of IA gemcitabine and RenovoCath. The FDA has determined that RenovoGem will be regulated as, and if approved we expect will be reimbursed as, a new oncology drug product. To date, we have not obtained marketing approval for any drug/device combination product candidates, manufactured a commercial scale product or arranged for a third-party to do so on our behalf, or conducted sales and marketing activities necessary for successful product commercialization. Our limited operating history as a company makes any assessment of our future success and viability subject to significant uncertainty. As a result, it may be more difficult for investors to accurately predict our likelihood of success and viability than it could be if we had a longer operating history.
We will encounter expenses, difficulties, complications, delays, and other known and unknown factors and risks frequently experienced by clinical stage biopharmaceutical companies in rapidly evolving fields. We also may need to transition from a company with a research and clinical development focus to a company capable of supporting commercial activities. We have not yet demonstrated an ability to successfully overcome such risks and difficulties, or to make such a transition. If we do not adequately address these risks and difficulties or successfully make such a transition, our business will suffer.
We have incurred significant net losses in each period since inception, and we expect to continue to incur net losses for the foreseeable future.
We are a clinical stage company and have incurred significant losses since our formation. As of December 31, 2023, we have an accumulated total deficit of approximately $41.4 million. For the fiscal years ended December 31, 2023 and 2022, we had net losses of approximately $10.2 million and $9.9 million, respectively. To date, we have experienced negative cash flow from the development of our product candidate, RenovoGem, our platform technology, TAMP, and our RenovoCath delivery system. We have not generated any revenue from operations, and we expect to incur substantial net losses for the foreseeable future as we seek to further develop and commercialize RenovoGem and expand our pipeline of product candidates. Because of the numerous risks and uncertainties associated with developing and commercializing RenovoGem, we are unable to predict the extent of any future losses or when we will attain profitability, if ever. Investors in our common stock must carefully consider the substantial challenges, risks and uncertainties inherent in the attempted development and commercialization of RenovoGem. We may never successfully commercialize RenovoGem, and our business may not be successful.
Our product candidates will require substantial additional development time and resources before we will be able to receive regulatory approvals, if any, and, if approved, to begin generating revenue from product sales. As a result, we expect that will be several years, if ever, before we receive approval to commercialize a product and generate revenue from product sales. Even if we succeed in receiving marketing approval for and commercializing one or more of our product candidates, we expect that we will continue to incur substantial expenses and increasing operating losses in the foreseeable future. The amount of our future net losses will depend, in part, on the level of our future expenditure and revenue. Moreover, our net losses may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. If we are unable to generate significant revenue from RenovoGem or attain profitability, we will not be able to sustain operations.
| 45 |
We anticipate that our expenses will increase substantially if and as we:
| ● | continue our research and discovery activities; | |
| ● | continue the development of our proprietary technology platform; | |
| ● | progress our current and any future product candidates through preclinical and clinical development; | |
| ● | initiate and conduct additional preclinical, clinical, or other studies for our product candidates; | |
| ● | work with our contract manufacturing organizations to manufacture RenovoCath and our other product candidates for our clinical trials; | |
| ● | change or add additional contract manufacturers or suppliers; | |
| ● | seek regulatory approvals and marketing authorizations for our product candidates; | |
| ● | establish sales, marketing, and distribution infrastructure to commercialize any products for which we obtain approval; | |
| ● | take steps to seek protection of our intellectual property and defend our intellectual property against challenges from third parties; | |
| ● | obtain, expand, maintain, protect, and enforce our intellectual property portfolio; | |
| ● | pursue any licensing or collaboration opportunities; | |
| ● | attract, hire, and retain key and qualified personnel including clinical, scientific, management, and administrative personnel; | |
| ● | provide additional internal infrastructure to support our continued research and development operations and any planned commercialization efforts in the future; | |
| ● | experience any delays or encounter other issues related to our operations; | |
| ● | implement operations, financial, and management information systems; | |
| ● | meet the requirements and demands of being a public company; and | |
| ● | defend against any product liability claims or other lawsuits related to our products. |
Our prior operating losses and expected future net losses have had and will continue to have an adverse effect on our stockholders’ equity, working capital, and our ability to fund our development efforts and achieve and maintain profitability. In any particular period, our operating results could be below the expectations of securities analysts or investors, or such analysts or investors could perceive these results to be negative, which could have a substantial adverse effect on the price of our common stock.
| 46 |
We will need to raise substantial additional capital to develop and commercialize RenovoGem, and our failure to obtain funding when needed may force us to delay, reduce or eliminate our product development programs or collaboration efforts. If we do not obtain adequate and timely funding, we may not be able to continue as a going concern.
As of December 31, 2023, we had cash and cash equivalents of $1.2 million. Due to our recurring operating losses and the expectation that we will continue to incur net losses in the future, we will be required to raise additional capital to complete the development and commercialization of our product candidates. We have historically financed our operations primarily through private sales of our equity, debt financing and the sale of common stock and warrants in our initial public offering (“IPO”). To raise additional capital, we may seek to sell additional equity and/or debt securities, obtain a credit facility or other loan or enter into collaborations, licenses or other similar arrangements, which we may not be able to do on favorable terms, or at all. For example, we have filed an omnibus shelf registration statement on Form S-3 that provides for aggregate offerings of up to $50 million of our securities subject to various limitations, including limited sales in any twelve-month period while we are subject to the “baby-shelf” rules. On April 3, 2023, we completed the Registered Direct Offering under our shelf registration statement on Form S-3 for the purchase and sale of 1,557,632 shares of our common stock (or pre-funded common stock warrants) at a purchase price of $3.21 per share of common stock (or pre-funded common stock warrants) to a certain institutional investor. Additionally, in a concurrent private placement, we issued to the investor unregistered warrants to purchase up to 1,947,040 shares of our common stock. The aggregate gross proceeds from the March 2023 Offering were approximately $5 million before deducting placement fees and other offering expenses. We also have filed a registration statement on Form S-1 to register the cash exercise of our outstanding warrants, with such cash exercise only expected to occur when the trading price of our common stock is in excess of the $10.80 per share exercise price of our outstanding warrants.
On January 26, 2024, we completed a private placement to 92 accredited investors with gross proceeds of $6.1 million. The private placement included issuing 6,133,414 shares of our common stock including 6,133,414 million common stock warrants, expiring five years from the date, January 26, 2024, to purchase additional common shares. In connection with the private placement, we entered into a placement agent agreement, as additional compensation to the placement agent, and issued 511,940 common stock warrants expiring five years from the settlement date.
Our ability to obtain additional financing will be subject to a number of factors, including market conditions, fluctuations in interest rates, our operating performance and investor sentiment. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue the development and/or commercialization of our product candidates, restrict or cease our operations or obtain funds by entering into agreements on unfavorable terms. Failure to obtain additional capital on acceptable terms, or at all, would result in a material and adverse impact on our operations. As a result, there is substantial doubt about our ability to operate as a going concern and we estimate that our current capital resources will be sufficient to fund our operating expenses and capital expenditure requirements through the fourth quarter of 2024.
Our financial statements as of December 31, 2023 have been prepared on a going concern basis and do not include any adjustments that may result from the outcome of this uncertainty. If we fail to raise additional working capital, or do so on commercially unfavorable terms, it would materially and adversely affect our business, prospects, financial condition and results of operations, and we may be unable to continue as a going concern. As a result, there is substantial doubt about our ability to operate as a going concern. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms, if at all. If we are unable to continue as a going concern, we might have to liquidate our assets and the value we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements, and our shareholders may lose their entire investment in our common stock.
| 47 |
We may consider strategic alternatives in order to maximize stockholder value, including financing, strategic alliances, and licensing arrangements. We may not be able to identify or consummate any suitable strategic alternatives and any consummated strategic alternatives may not be successful.
We may consider all strategic alternatives that may be available to us to maximize stockholder value, including financing, strategic alliances, and licensing arrangements. Our exploration of various strategic alternatives may not result in any specific action or transaction. To the extent that this engagement results in a transaction, our business objectives may change depending upon the nature of the transaction. There can be no assurance that we will enter into any transaction as a result of the engagement. Furthermore, if we determine to engage in a strategic transaction, we cannot predict the impact that such strategic transaction might have on our operations or stock price. We also cannot predict the impact on our stock price if we fail to enter into a transaction.
In addition, we face significant competition in seeking appropriate strategic partners, and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our business activities because they may be deemed to be at too early of a stage of development for collaborative effort. Any delays in entering into new strategic partnership agreements harm our business prospects, financial condition and results of operations.
If we license or acquire products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture. We cannot be certain that, following a strategic transaction, license, or acquisition, we will achieve the results, revenue or specific net income that justifies such transaction.
Risks Related to the Discovery, Development, and Commercialization of Our Product Candidates
Our product candidates’ commercial viability remains subject to current and future preclinical studies, clinical trials, regulatory approvals, and the risks generally inherent in the development of a pharmaceutical product candidate. If we are unable to successfully advance or develop our product candidate, our business will be materially harmed.
In the near-term, failure to successfully advance the development of any of our product candidates may have a material adverse effect on us. To date, we have not successfully developed or commercially marketed, distributed, or sold any product candidate. The success of our business depends primarily upon our ability to successfully advance the development of our current and future product candidates through preclinical studies and clinical trials, have the product candidates approved for sale by the FDA or regulatory authorities in other countries, and ultimately have the product candidates successfully commercialized by us or a commercial partner. We cannot assure you that the results of our ongoing preclinical studies or clinical trials will support or justify the continued development of our product candidates, or that we will receive regulatory approval from the FDA, or similar regulatory authorities in other countries, to advance the development of our product candidates.
Our product candidates must satisfy rigorous regulatory standards of safety and efficacy before we can advance or complete their clinical development, or before they can be approved for sale. To satisfy these standards, we must engage in expensive and lengthy preclinical studies and clinical trials, develop acceptable manufacturing processes, and obtain regulatory approval. Despite these efforts, the FDA could delay, limit or deny approval of a product candidate for many reasons, including because the FDA:
| ● | may not deem our product candidate to be safe and effective; |
| 48 |
| ● | determines that the product candidate does not have an acceptable benefit-risk profile; | |
| ● | may not agree that the data collected from preclinical studies and clinical trials are acceptable or sufficient to support the submission of an NDA or other submission or to obtain regulatory approval, and may impose requirements for additional preclinical studies or clinical trials; | |
| ● | may determine that adverse events experienced by participants in our clinical trials represent an unacceptable level of risk; | |
| ● | may determine that population studied in the clinical trial may not be sufficiently broad or representative to assure safety in the full population for which we seek approval; | |
| ● | may disagree regarding the formulation, labeling and/or the specifications; | |
| ● | may not approve the manufacturing processes associated with our product candidate or may determine that a manufacturing facility does not have an acceptable compliance status; | |
| ● | may conclude there are chemistry, manufacturing and controls issues that preclude approval of the NDA; | |
| ● | may conclude that the drug substance or drug product manufacturing process is not in a state of control or does not meet cGMP or all the regulatory requirements; | |
| ● | may conclude that the medical device manufacturing process for the drug/device combination product candidate is not in a state of control or does not meet all the regulatory requirements; | |
| ● | may change approval policies or adopt new regulations; or | |
| ● | may not file a submission due to, among other reasons, the content or formatting of the submission. |
If we experience delays in obtaining approval or if we fail to obtain approval of RenovoGem, our commercial prospects will be harmed and our ability to generate revenues will be materially impaired which would adversely affect our business, prospects, financial condition and results of operations.
We cannot assure you that the results of late-stage clinical trials will be favorable enough to support the continued development of our product candidates. A number of companies in the pharmaceutical and biopharmaceutical industries have experienced significant delays, setbacks and failures in all stages of development, including late-stage clinical trials, even after achieving promising results in preclinical testing or early-stage clinical trials. Accordingly, results from completed preclinical studies and early-stage clinical trials of our product candidates may not be predictive of the results we may obtain in later-stage trials. Furthermore, even if the data collected from preclinical studies and clinical trials involving our product candidates demonstrate a favorable safety and efficacy profile, such results may not be sufficient to support the submission of an NDA to obtain regulatory approval from the FDA in the U.S., or other similar regulatory agencies in other jurisdictions, which is required to market and sell the product. Even if we are successful in obtaining approval in one jurisdiction, we may not be successful in obtaining approval in any other jurisdictions. If we are unable to obtain approval for our product candidates in multiple jurisdictions, our business, financial condition, results of operations and our growth prospects could be negatively affected.
Our product candidates will require significant additional research and development efforts, the commitment of substantial financial resources, and regulatory approvals prior to advancing into further clinical development or being commercialized by us or collaborators. We cannot assure you that our product candidates will successfully progress through the drug development process or will result in commercially viable products. We do not expect our product candidates to be commercialized by us or collaborators for at least several years.
| 49 |
If we do not achieve our projected development goals in the timeframes we announce and expect, our stock price may decline.
From time to time, we estimate the timing of the anticipated accomplishment of various scientific, clinical, regulatory and other product development goals, which we sometimes refer to as milestones. These milestones may include the commencement or completion of scientific studies and clinical trials and the submission of regulatory filings. From time to time, we may publicly announce the expected timing of some of these milestones. All of these milestones are and will be based on numerous assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in some cases for reasons beyond our control. If we do not meet these milestones as publicly announced, our stock price may decline.
Our product candidates may exhibit undesirable side effects when used alone or in combination with other approved pharmaceutical products or investigational new drugs, which may delay or preclude further development or regulatory approval or limit their use if approved.
Throughout the drug development process, we must continually demonstrate the efficacy, safety and tolerability of our product candidates to obtain regulatory approval to further advance clinical development or to market them. Even if our product candidates demonstrate clinical efficacy, any unacceptable, adverse side effects or toxicities, when administered alone or in the presence of other pharmaceutical products, which can arise at any stage of development, may outweigh the potential benefits. In preclinical studies and clinical trials we have conducted to date, each of our product candidate’s tolerability profile is based on studies and trials that have involved a small number of subjects or patients over a limited period of time. We may observe adverse or significant adverse events or drug-drug interactions in future preclinical studies or clinical trial candidates, which could result in the delay or termination of development, prevent regulatory approval, or limit market acceptance if ultimately approved.
If the results of preclinical studies or clinical trials for our product candidates, including those that are subject to existing or future license or collaboration agreements, are unfavorable or delayed, we could be delayed or precluded from the further development or commercialization of our product candidates, which could materially harm our business.
To further advance the development of, and ultimately receive regulatory approval to sell, our product candidates, we must conduct extensive preclinical studies and clinical trials to demonstrate their safety and efficacy to the satisfaction of the FDA or similar regulatory authorities in other countries, as the case may be. Preclinical studies and clinical trials are expensive, complex, can take many years to complete, and have highly uncertain outcomes. Delays, setbacks, or failures can occur at any time, or in any phase of preclinical or clinical testing, and can result from concerns about safety or toxicity, a lack of demonstrated efficacy or superior efficacy over other similar products that have been approved for sale or are in more advanced stages of development, poor study or trial design, and issues related to the formulation or manufacturing process of the materials used to conduct the trials. The results of prior preclinical studies or clinical trials are not necessarily predictive of the results we may observe in later stage clinical trials. In many cases, product candidates in clinical development may fail to show desired safety, efficacy or tolerability characteristics despite having favorably demonstrated such characteristics in preclinical studies or earlier stage clinical trials.
In addition, we may experience numerous unforeseen events during, or as a result of, preclinical studies and the clinical trial process, which could delay or impede our ability to advance the development of, receive regulatory approval for, or commercialize our product candidate, including, but not limited to:
| ● | communications with the FDA, or similar regulatory authorities in different countries, regarding the scope or design of a trial or trials; | |
| ● | regulatory authorities, including an Institutional Review Board (“IRB”) or Ethical Committee (“EC”), not authorizing us to commence or conduct a clinical trial at a prospective trial site; | |
| ● | enrollment in our clinical trials being delayed, or proceeding at a slower pace than we expected, because our clinical trial sites have staffing shortages or are unable to recruit/retain qualified staff, or we have difficulty recruiting patients, including as a result of competing clinical trials, or participants dropping out of our clinical trials at a higher rate than we anticipated; |
| 50 |
| ● | our third-party contractors, upon whom we rely for conducting preclinical studies, clinical trials and manufacturing of our trial materials, may fail to comply with regulatory requirements, fail to meet their contractual obligations to us in a timely manner, or terminate their relationship with us; | |
| ● | having to suspend or ultimately terminate our clinical trials if participants are being exposed to unacceptable health or safety risks; | |
| ● | IRBs, ECs, or regulators requiring that we hold, suspend or terminate our preclinical studies and clinical trials for various reasons, including non-compliance with regulatory requirements, including the effects of recent variants; and | |
| ● | the supply or quality of drug material or the supply of our RenovoCath delivery service necessary to conduct our preclinical studies or clinical trials being insufficient, inadequate or unavailable. |
Even if the data collected from preclinical studies or clinical trials involving our product candidates demonstrate a favorable safety and efficacy profile, such results may not be sufficient to support the submission of an NDA to obtain regulatory approval from the FDA in the U.S., or other similar foreign regulatory authorities in foreign jurisdictions, which is required to market and sell the product.
We may be required to perform additional or unanticipated clinical trials to obtain approval or be subject to additional post-marketing testing requirements to maintain regulatory approval. In addition, regulatory authorities may withdraw their approval of a product or impose restrictions on our distribution, such as in the form of a Risk Evaluation and Mitigation Strategy, or REMS. The failure to obtain timely regulatory approval of product candidates, any product marketing limitations or a product withdrawal would materially and adversely affect our business, results of operations and financial condition.
Interim, preliminary or topline data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, we may publish interim, preliminary or topline data from clinical trials. Interim data from clinical trials that we may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data becomes available. Preliminary or topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary or topline data previously published. As a result, interim, preliminary and topline data should be viewed with caution until the final data are available. Adverse differences between interim, preliminary or topline data and final data could significantly harm our reputation and business prospects. Moreover, preliminary, interim and topline data are subject to the risk that one or more of the clinical outcomes may materially change as more patient data become available when patients mature on study, patient enrollment continues or as other ongoing or future clinical trials with a product candidate further develop. Past results of clinical trials may not be predictive of future results. In addition, the information we choose to publicly disclose regarding a particular study or clinical trial is based on what is typically more extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure. Any information we determine not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product candidate or our business. Similarly, even if we are able to complete our planned and ongoing preclinical studies and clinical trials of our product candidates according to our current development timeline, the positive results from such preclinical studies and clinical trials of our product candidates may not be replicated in subsequent preclinical studies or clinical trial results.
| 51 |
If third party vendors upon whom we intend to rely on to conduct our preclinical studies or clinical trials do not perform or fail to comply with strict regulations, these studies or trials of our product candidate may be delayed, terminated, or fail, or we could incur significant additional expenses, which could materially harm our business.
We have limited resources dedicated to designing, conducting, and managing preclinical studies and clinical trials. We intend to rely on third parties, including clinical research organizations, consultants, and principal investigators, to assist us in designing, managing, monitoring and conducting our preclinical studies and clinical trials. We intend to rely on these vendors and individuals to perform many facets of the drug development process, including certain preclinical studies, the recruitment of sites and patients for participation in our clinical trials, maintenance of good relations with the clinical sites, and ensuring that these sites are conducting our trials in compliance with the trial protocol, including safety monitoring and applicable regulations. If these third parties fail to perform satisfactorily, or do not adequately fulfill their obligations under the terms of our agreements with them, or terminate their relationship with us, we may not be able to enter into alternative arrangements without undue delay or additional expenditures, and therefore the preclinical studies and clinical trials of our product candidate may be delayed or prove unsuccessful. For example, the investigators we currently use for our clinical trials are not our employees and we cannot control the amount or timing of resources that they devote to our programs. If these investigators fail to devote sufficient time and resources to our clinical trial, fail to enroll patients as rapidly as expected, or otherwise do not perform in a satisfactory manner, we may make elect to close such clinical trial site, which may increase our expenses, require additional attention from our clinical team and delay our clinical trial timeline and regulatory approval. Further, the FDA, or other similar foreign regulatory authorities, may inspect some of the clinical sites participating in our clinical trials in the U.S., or our third-party vendors’ sites, to determine if our clinical trials are being conducted according to good clinical practice, or GCP. If we or the FDA determine that our third-party vendors are not in compliance with, or have not conducted our clinical trials according to, applicable regulations we may be forced to delay, repeat, or terminate such clinical trials.
We have limited capacity for recruiting and managing clinical trials, which could impair our timing to initiate or complete clinical trials of our product candidate and materially harm our business.
We have limited capacity to recruit and manage the clinical trials necessary to obtain FDA approval or approval by other regulatory authorities. By contrast, larger pharmaceutical and biopharmaceutical companies often have substantial staff with extensive experience in conducting clinical trials with multiple product candidates across multiple indications. In addition, they may have greater financial resources to compete for the same clinical investigators and patients that we are attempting to recruit for our clinical trials. If potential competitors are successful in completing drug development for their product candidates and obtain approval from the FDA, they could limit the demand to participate in clinical trials of our product candidates. As a result, we may be at a competitive disadvantage that could delay the initiation, recruitment, timing, and completion of our clinical trials, as well as obtaining regulatory approvals, if at all, for our product candidates.
We, and our collaborators, if any, must comply with extensive government regulations in order to advance our product candidates through the development process and ultimately obtain and maintain marketing approval for our products in the U.S. and abroad.
The product candidates that we, or our collaborators, are developing or may develop require regulatory approval to advance through clinical development and to ultimately be marketed and sold and are subject to extensive and rigorous domestic and foreign government regulation. In the U.S., the FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record-keeping, labeling, storage, approval, advertising, promotion, sale, and distribution of pharmaceutical and biopharmaceutical products. Our product candidates are also subject to similar regulation by foreign governments to the extent we seek to develop or market them in those countries. We, or our collaborators, must provide the FDA and foreign regulatory authorities, if applicable, with preclinical and clinical data, as well as data supporting an acceptable manufacturing process, that appropriately demonstrate each of our product candidate’s safety and efficacy before it can be approved for the targeted indications. Our product candidates have not been approved for sale in the U.S. or any foreign market, and we cannot predict whether we or our collaborators will obtain regulatory approval for any product candidates we are developing or plan to develop. The regulatory review and approval process can take many years, is dependent upon the type, complexity, novelty of, and medical need for the product candidate, requires the expenditure of substantial resources, and involves post-marketing surveillance and vigilance and potentially post-marketing studies or Phase IV clinical trials. In addition, we or our collaborators may encounter delays in, or fail to gain, regulatory approval for any of our product candidates based upon additional governmental regulation resulting from future legislative, administrative action or changes in the FDA’s or other similar foreign regulatory authorities’ policy or interpretation during the period of product development. Delays or failures in obtaining regulatory approval to advance any of our product candidates through clinical development, and ultimately to commercialize them, may:
| ● | adversely impact our ability to raise sufficient capital, if at all, to fund the development of our product candidates; |
| 52 |
| ● | adversely affect our ability to further develop or commercialize our product candidates; | |
| ● | diminish any competitive advantages that we or our collaborators may have or attain; or | |
| ● | adversely affect the receipt of potential milestone payments and royalties from collaborators, if any, from the sale of our products or product revenues in the future. |
Furthermore, any regulatory approvals, if granted, may later be withdrawn. If we or our collaborators fail to comply with applicable regulatory requirements at any time, or if post-approval safety concerns arise, we or our collaborators may be subject to restrictions or a number of actions, including:
| ● | delays, suspension, or termination of clinical trials related to our product candidates; | |
| ● | refusal by regulatory authorities to review pending applications or supplements to approved applications; | |
| ● | product recalls or seizures; | |
| ● | suspension of manufacturing; | |
| ● | withdrawals of previously approved marketing applications; or | |
| ● | fines, civil penalties, and criminal prosecutions. |
Additionally, at any time we or our collaborators may voluntarily suspend or terminate the preclinical or clinical development of a product candidate, or withdraw any approved product from the market if we believe that it may pose an unacceptable safety risk to patients, or if the product candidate or approved product no longer meets our business objectives. The ability to develop or market a pharmaceutical product outside of the U.S. is contingent upon receiving appropriate authorization from the respective foreign regulatory authorities. Foreign regulatory approval processes typically include many, if not all, of the risks and requirements associated with the FDA regulatory process for drug development and may include additional risks. Additionally, results acceptable to support approval in one jurisdiction may be deemed inadequate by another regulatory authority to support regulatory approval in that other jurisdiction.
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Our product candidates may not prove to be safe and efficacious in clinical trials and may not meet all the applicable regulatory requirements needed to receive regulatory approval. To receive regulatory approval for the commercialization of our product candidates, we must conduct, at our own expense, extensive preclinical testing and clinical trials to demonstrate safety and efficacy of our product candidate for the intended indication of use. Clinical testing is expensive, can take many years to complete, if at all, and its outcome is uncertain. Failure can occur at any time during the clinical trial process.
The results of preclinical studies and early clinical trials of new drugs do not necessarily predict the results of later-stage clinical trials. The design of our clinical trials is based on many assumptions about the expected effects of our product candidate, and if those assumptions are incorrect, they may not produce statistically significant results. Preliminary results may not be confirmed on full analysis of the detailed results of a clinical trial. Product candidates in later stages of clinical development may fail to show safety and efficacy sufficient to support intended use claims despite having progressed through earlier clinical testing. The data collected from clinical trials of our product candidates may not be sufficient to support the filing of an NDA or to obtain regulatory approval in the United States or elsewhere. Because of the uncertainties associated with drug development and regulatory approvals, we cannot determine if or when we will have an approved product for commercialization or achieve sales or profits.
| 53 |
Delays in clinical testing could result in increased costs to us and delay our ability to generate revenue.
We may experience delays in clinical testing of our product candidates. We do not know whether planned clinical trials will begin on time, will need to be redesigned or will be completed on schedule, if at all. From time to time, based on our experience with a clinical trial, we may amend the clinical trial protocol to address any issues that we observe as the trial is progressing, including in response to various factors impacting safety and the data collected, or we may be required to make certain changes in response to issues raised by the FDA, IRB, other regulatory authorities, investigators or clinical sites. Protocol amendments are subject to IRB and regulatory approval before we implement material changes, can result in additional costs, require additional data or participants, and may negatively impact the timelines for the trial. For example, in December 2021, we amended the protocol for our Phase III clinical trial to only allow SBRT patients during the induction phase of the study, as we observed a higher drop-out rate for patients on IMRT. As part of this change, we initiated a review of the statistical considerations for the study and in June 2022, submitted a Modified SAP to the FDA. We submitted a protocol amendment to the FDA in the second half of 2022 to reflect the changes in the Modified SAP. Under the modified Phase III clinical trial protocol and the Modified SAP, we plan to (i) analyze only patients receiving SBRT, consistent with the protocol change made in December 2021, (ii) include a second interim analysis, (iii) change the total number of SBRT patients randomized in the study to 114 (a reduction from the original 200 patients), with a total of 86 deaths from SBRT patients, including all deaths from SBRT patients enrolled in the study before the submission of the Modified SAP, and (iv) repower the study from 90% to 80%, which is commonly used in clinical trials. In August 2023, we received comments from the FDA on the Modified SAP, which we responded to in September 2023 Based on the FDA’s feedback, we plan to submit the revised SAP and a protocol amendment to reflect the changes in the statistical analysis for the study. Such amendment and Modified SAP are subject to IRB and regulatory review that may result in additional costs and may negatively impact the timelines for the trial. We cannot provide assurance that the FDA will not raise any objections or disagree with our Modified SAP or the protocol amendments, including our proposed SAP or how we interpret the data. We can provide no assurance on the timing of any of our interim analyses or when we will complete our Phase III study, if at all, or the outcome of the study. Disclosure of findings from our interim analyses before the completion of the trial may also impact the enrollment or retention of patients in our ongoing clinical trial. The changes in our study protocol may limit the clinical trial sites that can participate in our study, impact enrollment, and delay regulatory approval. We may be required to expand the size of our study, increase the power level, or make other changes that can delay our clinical timelines and delay regulatory approval. Further, to the extent protocol amendments impact the data needed to support our proposed indication, the indication that is ultimately approved by the FDA or other regulatory authorities may be narrower than the indication initially sought. The FDA and other regulatory authorities may also impose other restrictions in our proposed labeling, which could have a material adverse effect on the prospects of our product candidates, if approved, and our business.
Clinical trials can be delayed for a variety of reasons, including pandemics, delays in obtaining regulatory approval to commence a clinical trial, in securing clinical trial agreements with prospective sites with acceptable terms, in obtaining IRB approval to conduct a clinical trial at a prospective site, in recruiting patients to participate in a clinical trial or in obtaining sufficient supplies of clinical trial materials, including RenovoCath. Many factors affect patient enrollment, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the clinical trial, the existing body of safety and efficacy data with respect to the study drug, competing clinical trials, new drugs approved for the conditions we are investigating, clinicians’ and patients’ perceptions of the potential advantages and side effects of the product candidates being studied in relation to other available therapies and product candidates. Clinical investigators will need to decide whether to offer their patients enrollment in clinical trials of our product candidate versus treating these patients with commercially available drugs that have established safety and efficacy profiles. Any delays in completing our clinical trials will increase our costs, slow down our product development, timeliness and approval process, and delay our ability to generate revenue.
| 54 |
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable but it typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that any of our existing product candidates or any product candidate we may seek to develop in the future may never obtain regulatory approval.
Our product candidates could fail to receive regulatory approval for many reasons, including the following:
| ● | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; | |
| ● | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; | |
| ● | the results of clinical trials may not meet the level of statistical significance required for approval by the FDA or comparable foreign regulatory authorities; | |
| ● | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; | |
| ● | the data collected from clinical trials of our product candidates may not be sufficient to support the submission of an NDA or other submission or to obtain regulatory approval in the United States or elsewhere; | |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; or | |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market our product candidates, which would significantly harm our business, results of operations, prospects and our underlying stock price.
In addition, even if we were to obtain approval, regulatory authorities may approve our product candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Any of the foregoing scenarios could materially harm the commercial prospects for any of our product candidates.
We have not previously submitted an NDA to the FDA, nor similar drug approval filings to comparable foreign authorities, for our product candidates, and we cannot be certain that our product candidates will be successful in clinical trials or receive regulatory approval. Further, our product candidates may not receive regulatory approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our product candidates, we may not be able to continue our operations. Even if we successfully obtain regulatory approvals to market one or more of our product candidates, our revenues will be dependent on many factors including the size of the markets in the territories for which we gain regulatory approval and have commercial rights. If the markets for patients that we are targeting for our product candidates are not as significant as we estimate, we may not generate significant revenues from sales of such products, if approved.
We plan to seek regulatory approval and to commercialize our product candidates, directly or with collaborators in the United States, the European Union, and other foreign countries which we have not yet identified. While the scope of regulatory approval is similar in other countries, to obtain separate regulatory approval in many other countries we must comply with numerous and varying regulatory requirements of such countries regarding the safety and efficacy, among other things, of clinical trials and commercial sales, pricing, and distribution of our product candidates, and we cannot predict success in these jurisdictions.
| 55 |
We may be required to suspend or discontinue clinical trials due to unexpected side effects or other safety risks that could preclude approval of our product candidates.
Our clinical trials may be suspended at any time for a number of reasons. For example, we may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to the clinical trial patients. In addition, the FDA or other regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the clinical trial patients.
Administering our product candidates to humans may produce undesirable side effects. These adverse side effects could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities denying further development or approval of any of our product candidates for any or all targeted indications. Ultimately, our product candidates may prove to be unsafe for human use. Moreover, we could be subject to significant liability if any volunteer or patient suffers, or appears to suffer, adverse health effects as a result of participating in our clinical trials. Prosecution, enforcement actions, damages or adverse media coverage related to such events, if any, will likely result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees. As a general matter, such events could damage our reputation, brand, international activities, business, prospects, operating results and financial condition.
If we fail to comply with healthcare regulations, we could face substantial enforcement actions, including civil and criminal penalties and our business, operations and financial condition could be adversely affected.
Our business operations and activities may be directly or indirectly subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. The product candidates that we are developing are highly regulated, and there can be no assurance that the regulatory environment in which we operate will not change significantly and adversely in the future. If we begin commercializing any products cleared or approved by the FDA in the United States, our exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. Our current and future arrangements with healthcare professionals, clinical investigators, CROs, third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. In addition, we may be subject to laws of the federal government and state governments in which we conduct our business relating to privacy, data protection and data security with respect to patient information.
As a developer of drug/device combination products and a proprietary drug delivery device, certain federal and state healthcare laws and regulations pertaining to fraud and abuse, false claims, transparency and patients’ privacy rights are and will be applicable to our business. We could be subject to healthcare fraud and abuse laws, transparency and privacy laws of both the federal government and the states in which we conduct our business and private “qui tam” actions brought by individual whistleblowers on behalf of the federal or state governments. The scope and enforcement of each of the laws applicable to our business and products are uncertain and subject to rapid change in the current environment of healthcare reform. The laws include:
| ● | the federal healthcare program anti-kickback law, which prohibits, among other things, persons from soliciting, receiving or providing remuneration, directly or indirectly, to induce either the referral of an individual, for an item or service or the purchasing or ordering of a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; | |
| ● | federal false claims laws which prohibit, among other things, individuals, or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us which provide coding and billing information to customers; |
| 56 |
| ● | the federal Health Insurance Portability and Accountability Act of 1996, which prohibits executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; | |
| ● | the federal Open Payments program under the Physician Payments Sunshine Act, created under Section 6002 of the ACA and its implementing regulations, which requires certain manufacturers of covered drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) and applicable group purchasing organizations to report annually to CMS information related to payments or other transfers of value made in the previous year to covered recipients, including physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician healthcare professionals (such as physician assistants and nurse practitioners, among others), and teaching hospitals, and information regarding ownership and investment interests held by physicians (as defined above) and their immediate family members; | |
| ● | the federal Food, Drug, and Cosmetic Act, which among other things, strictly regulates drug manufacturing and product marketing, prohibits manufacturers from marketing drug products for off-label use and regulates the distribution of drug samples; | |
| ● | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; | |
| ● | federal government drug price reporting laws, changed by the ACA to, among other things, increase the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program and offer such rebates to additional populations, that require us to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement or discounts on our marketed drugs (participation in these programs and compliance with the applicable requirements may subject us to potentially significant discounts on our product candidates, increased infrastructure costs, and potentially limit our ability to offer certain marketplace discounts); | |
| ● | the Foreign Corrupt Practices Act, a United States law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); | |
| ● | analogous state and foreign laws and regulations, including: state anti-kickback and false claims laws which may apply to our business practices, including, but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by state governmental and non-governmental third-party payors, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government; state laws that require drug manufacturers to track gifts and other remuneration and items of value provided to healthcare professionals and entities; state and local laws that require the registration of pharmaceutical sales representatives; and state laws that require drug manufacturers to report information relating to pricing and marketing information; and | |
| ● | state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by federal laws, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws. If our operations are found to be in violation of any of the laws described above or any governmental regulations that apply to us, we may be subject to greater liabilities, penalties, including civil and criminal penalties, damages, fines, and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert management’s attention from the operation of our business.
| 57 |
If any of the physicians or other providers or entities with whom we expect to do business, is found not to be in compliance with applicable laws, it may be subject to significant criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
We are also subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations which can harm our business. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other partners from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties for clinical trials outside of the United States, to sell our products abroad once we enter a commercialization phase, or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We may have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other partners, even if we do not explicitly authorize or have actual knowledge of such activities. Any violation of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
If we are unable to satisfy regulatory requirements, we may not be able to commercialize our product candidates.
We need FDA approval prior to marketing our product candidates in the United States. This approval process is lengthy and subject to extensive governmental regulations and given the unpredictability of the results of clinical trials, our failure to obtain regulatory approval from the FDA to market any of our product candidates would significantly harm our business, results of operations and prospects. Any delay or failure in seeking or obtaining required approvals from the FDA to market any of our product candidates would have a material and adverse effect on our ability to sell our product candidates in the United States and to generate revenue from any such candidates we are developing and for which we are seeking approval.
The FDA’s review and approval process, including among other things, evaluation of preclinical studies and clinical trials of a product candidate as well as the manufacturing process and facility, is lengthy, expensive, and uncertain. To receive approval, we must, among other things, demonstrate with substantial evidence from well-designed and well-controlled preclinical testing and clinical trials that the product candidates are both safe and effective for each indication for which approval is sought. Satisfaction of these requirements typically takes several years, and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product. We cannot predict if or when we will submit an NDA for approval for any of our product candidates currently under development. Any approvals we may obtain may not cover all of the clinical indications for which we are seeking approval or may contain significant limitations on the conditions of use.
The FDA has substantial discretion in the NDA review process and may either refuse to file our NDA for substantive review or may decide that our data is insufficient to support approval of our product candidates for the claimed intended uses. Following any regulatory approval of our product candidates, we will be subject to continuing regulatory obligations such as safety reporting, required and additional post marketing obligations, and regulatory oversight of promotion and marketing. Even if we receive regulatory approvals for any of our product candidates, the FDA may subsequently seek to withdraw approval of our NDA if we determine that new data or a reevaluation of existing data show the product is unsafe for use under the conditions of use upon the basis of which the NDA was approved, or based on new evidence of adverse effects or adverse clinical experience, or upon other new information. If the FDA does not file or approve our NDA or withdraws approval of our NDA, the FDA may require that we conduct additional clinical trials, preclinical or manufacturing studies and submit that data before it reconsiders our application. Depending on the extent of these or any other requested studies, approval of any applications that we submit may be delayed by several years, may require us to expend more resources than we have available, or may never be obtained at all. In addition, we have obtained FDA clearance for our RenovoCath delivery system, which is subject to FDA medical device regulations, including the Quality System Regulation. In the event adverse events arise with respect to the RenovoCath delivery system, the FDA could revoke its clearance which would have a material adverse effect on our business.
| 58 |
We will also be subject to a wide variety of foreign regulations governing the development, manufacture, and marketing of our products to the extent we seek regulatory approval to develop and market any of our product candidates in a foreign jurisdiction. As of the date hereof we have not identified any foreign jurisdictions which we intend to seek approval from. Whether or not FDA approval has been obtained, approval of a product candidate by the comparable regulatory authorities of foreign countries must still be obtained prior to marketing the product candidate in those countries. The approval process varies, and the time needed to secure approval in any region such as the European Union or in a country with an independent review procedure may be longer or shorter than that required for FDA approval. We cannot assure you that clinical trials conducted in one country will be accepted by other countries or that an approval in one country or region will result in approval elsewhere.
Even after approval, we are subject to extensive regulations. The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. The FDA and foreign counterparts enforce these regulatory requirements through, among other means, periodic unannounced inspections and periodic reviews of public marketing and promotion materials. We do not know whether we will be found compliant in connection with any future FDA or foreign counterparts’ inspections or reviews. Failure to comply with applicable regulations could jeopardize our ability to sell our products and result in enforcement actions such as: warning letters; untitled letters; fines; injunctions; civil penalties; termination of distribution; recalls or seizures of products; delays in the introduction of products into the market; total or partial suspension of production; refusal to grant future clearances, approvals, or certifications; withdrawals or suspensions of current approvals or certifications, resulting in prohibitions on sales of our products; and in the most serious cases, criminal penalties.
We have received Orphan Drug Designation for RenovoGem for two rare diseases: pancreatic cancer and cholangiocarcinoma. We may seek Orphan Drug Designation for future product candidates, but we may be unable to obtain such designation or to maintain the benefits associated with Orphan Drug Designation, including market exclusivity, which may cause our revenue, if any, to be reduced.
To date, we have secured FDA Orphan Drug Designation for RenovoGem in two rare diseases: pancreatic cancer and cholangiocarcinoma. Although we may seek Orphan Drug Designation for some or all of our other product candidates, we may never receive such designations. Under the Orphan Drug Act, the FDA may designate a drug product as an orphan drug if it is intended to treat a rare disease or condition, defined as a patient population of fewer than 200,000 in the United States, or a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. Orphan Drug Designation must be requested before submitting an NDA. In the United States, Orphan Drug Designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and application fee waivers. After the FDA grants Orphan Drug Designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA.
In addition, if a drug product receives the first FDA approval for an indication for which it has orphan designation, the drug product is entitled to orphan drug exclusivity, which means the FDA may not approve any other application to market the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan exclusivity or where the manufacturer is unable to assure sufficient product quantity for the orphan patient population. Exclusive marketing rights in the United States may also be unavailable if we seek approval for an indication broader than the orphan designation and may be lost if the FDA later determines that the request for designation was materially defective.
Even if we obtain Orphan Drug Designation, we may not be the first to obtain marketing approval for any particular indication within the orphan designation due to uncertainties associated with developing pharmaceutical products, which would have a material adverse effect on our operations, regulatory approval and ability to commercialize our product candidate. Further, even if we obtain orphan drug exclusivity for a product candidate, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug with the same active moiety for the same indication or use if the FDA concludes that the later drug is clinically superior or makes a major contribution to patient care. Orphan Drug Designation neither shortens the development time or regulatory review time of a product candidate nor gives the product candidate any advantage in the regulatory review or approval process or entitles the product candidate to priority review.
| 59 |
Further, in Catalyst Pharms., Inc. v. Becerra, 14 F.4th 1299 (11th Cir. 2021), the court disagreed with the FDA’s longstanding position that the orphan drug exclusivity only applies to the approved use or indication within an eligible disease. This decision created uncertainty in the application of the orphan drug exclusivity. On January 24, 2023, the FDA published a notice in the Federal Register to clarify that while the agency complies with the court’s order in Catalyst, the FDA intends to continue to apply its longstanding interpretation of the regulations to matters outside of the scope of the Catalyst order – that is, the agency will continue tying the scope of orphan-drug exclusivity to the uses or indications for which a drug is approved, which permits other sponsors to obtain approval of a drug for new uses or indications within the same orphan designated disease or condition that have not yet been approved. It is unclear how future litigation, legislation, agency decisions, and administrative actions will impact the scope of the orphan drug exclusivity.
If our product candidates are unable to compete effectively with marketed drugs targeting similar indications as our product candidates, our commercial opportunity will be reduced or eliminated.
We face competition generally from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. We are aware of a number of companies in Phase III clinical trials for the treatment of LAPC including AB Science, Angiodynamics, Bausch Health, Fibrogen, NovoCure, SynCore Biotechnology, BMS, and ViewRay. In addition, we are aware of a number of companies in Phase I and Phase II clinical trials for the treatment of LAPC including one interventional company, TriSalus Lifesciences. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize any products that are safer, more effective, have fewer side effects or are less expensive than our product candidates. These potential competitors compete with us in recruiting and retaining key and qualified scientific and management personnel, establishing clinical trial sites, and patient enrollment for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
If approved and commercialized, RenovoGem would compete with several currently approved prescription therapies for the treatment of LAPC and cholangiocarcinoma. To our knowledge, other potential competitors are in the earlier stages of development. If potential competitors are successful in completing drug development for their product candidates and obtain approval from the FDA, they could limit the demand for RenovoGem.
We expect that our ability to compete effectively will depend upon our ability to:
| ● | successfully identify and develop key points of product differentiation from currently available therapies; | |
| ● | successfully and timely complete clinical trials and submit for and obtain all requisite regulatory approvals in a cost-effective manner; | |
| ● | maintain a proprietary position for our products and manufacturing processes and other related product technology; | |
| ● | attract and retain key and qualified personnel; | |
| ● | develop relationships with physicians prescribing these products; and | |
| ● | build an adequate sales and marketing infrastructure for our products, if approved. |
| 60 |
Because we will be competing against significantly larger companies with established track records, we will have to demonstrate that, based on experience, clinical data, side-effect profiles and other factors, our products, if approved, are competitive with other products. If we are unable to compete effectively and differentiate our products from other marketed drugs, we may never generate meaningful revenue.
We may expend our limited resources to pursue one or more product candidates or indications within our product development strategy, which has and may continue to change over time, and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we intend to focus on developing product candidates for specific indications that we identify as most likely to succeed, in terms of their potential both to gain regulatory approval and to achieve commercialization. As a result, we may forego or delay the pursuit of opportunities with other product candidates or in other indications with greater commercial potential. Such resource allocation and strategic decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable product candidates. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing, or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to the product candidate.
If the manufacturers upon whom we rely fail to produce our product candidates, in the volumes that we require on a timely basis or fail to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the development and commercialization of our product candidates.
We do not currently possess internal manufacturing capacity. We plan to utilize the services of cGMP manufacturers, FDA inspected contract manufacturers to formulate and manufacture our preclinical and clinical supplies. Any curtailment in the availability of gemcitabine, or RenovoCath, the drug delivery device, however, could result in production or other delays with consequent adverse effects on us. In addition, because regulatory authorities must generally approve raw material sources for pharmaceutical products, changes in raw material suppliers may result in production delays or higher raw material costs.
We obtain our RenovoCath delivery system from a single source, which must be manufactured in accordance with the FDA Quality System Regulation (QSR). Gemcitabine is supplied from our clinical sites’ pharmacies and used off-label for IA use within our clinical study. We continue to pursue supply agreements for gemcitabine and our RenovoCath delivery system. We may be required to agree to minimum volume requirements, exclusivity arrangements or other restrictions with the contract manufacturers. We may not be able to enter into long-term agreements on commercially reasonable terms, or at all. If we change or add manufacturers, the FDA and comparable foreign regulators may require approval of the changes. Approval of these changes could require new testing by the manufacturer and compliance inspections to ensure the manufacturer is conforming to all applicable laws and regulations and cGMP.
The manufacture of pharmaceutical products, including drug/device combination products, requires significant expertise and capital investment, including the development of an acceptable formulation to support later-stage trials for our product candidates, advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products may encounter difficulties in production, particularly in scaling up production and reformulating the form of any of our product candidates. For drug/device combination products, ensuring compliance with both medical device and drug regulations exposes us to additional risks. These problems include difficulties with production costs and yields, quality control, including stability of the product and quality assurance testing, shortages of qualified personnel, as well as compliance with federal, state, and foreign regulations. Our contract manufacturers may also place a priority on the manufacture of their own products, or other customers’ products. In addition, any delay or interruption in the supply of clinical trial supplies could delay the completion of our clinical trials, increase the costs associated with conducting our clinical trials and, depending upon the period of delay, require us to commence new clinical trials at significant additional expense or to terminate a clinical trial.
| 61 |
We will be responsible for ensuring that our future contract manufacturers comply with the cGMP requirements of the FDA and other regulatory authorities from which we seek to obtain product approval. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. The approval process for NDAs includes an inspection of the manufacturer’s compliance with cGMP requirements. We will be responsible for regularly assessing a contract manufacturer’s compliance with cGMP requirements through record reviews and periodic audits and for ensuring that the contract manufacturer takes responsibility and corrective action for any identified deviations. Manufacturers of our product candidates may be unable to comply with these cGMP requirements and with other FDA and foreign regulatory requirements, if any.
While we will oversee compliance of our contract manufacturers, ultimately, we will not have control over our manufacturers’ compliance with these regulations and standards. A failure to comply with these requirements may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of any of our product candidates is compromised due to a manufacturers’ failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for or successfully commercialize any of our product candidates, and we may be held liable for any injuries sustained as a result. Any of these factors could cause a delay of clinical trials, regulatory submissions, approvals, or commercialization of RenovoGem or other product candidates, entail higher costs or result in us being unable to effectively commercialize any of our product candidates. Furthermore, if our manufacturers fail to deliver the required commercial quantities on a timely basis and at commercially reasonable prices, we may be unable to meet demand for any approved products and would lose potential revenues. There are also risks of our contract manufacturers failing to perform as agreed, terminating their relationship with us, experiencing the effects of any strikes or other work stoppages, or not remaining in the contract manufacturing business.
Our dependence on third-party suppliers subjects us to a number of risks that could negatively impact our ability to manufacture products and harm our business, including:
| ● | interruption of supply resulting from modifications to, or discontinuation of, a supplier’s operations; | |
| ● | delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s failure to produce components that consistently meet our quality specifications; | |
| ● | price fluctuations due to a lack of long-term supply arrangements with our suppliers for key components; | |
| ● | inability to obtain adequate supply in a timely manner or on commercially reasonable terms; | |
| ● | difficulty identifying and qualifying alternative suppliers for components in a timely manner; | |
| ● | inability of suppliers to comply with applicable provisions of the FDA’s QSR, cGMP regulations or other applicable laws or regulations enforced by the FDA or state regulatory authorities and foreign regulatory authorities; | |
| ● | inability to ensure the quality of products or components manufactured by third parties; | |
| ● | production delays related to the evaluation and testing of products and components from alternative suppliers and corresponding regulatory qualifications; | |
| ● | delays in delivery by our suppliers due to changes in demand from us or their other customers, or our suppliers prioritizing their other customers over us; and | |
| ● | an outbreak of disease or similar public health threat particularly as it may impact our supply chain. |
Although we require that our third-party suppliers provide our manufacturing partners with components that meet our specifications and comply with applicable provisions of the QSR, cGMP and other applicable legal and regulatory requirements in our agreements and contracts, there is a risk that our suppliers will not always act with our best interests in mind, and they may not always supply components that meet our requirements or supply components in a timely manner. Any interruption or delay in the supply of components or materials, or our inability to obtain components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to cancel orders or switch to competitive procedures. These events could harm our business and our operating results.
| 62 |
We may not be able to manufacture our product candidates in commercial quantities, which would prevent us from commercializing our product candidates.
To date, our product candidates have been manufactured in small quantities for preclinical studies and clinical trials. If any of our product candidates are approved by the FDA or comparable regulatory authorities in other countries for commercial sale, we will need to manufacture such product candidates in larger quantities. We may not be able to successfully increase the manufacturing capacity for our product candidates in a timely or economic manner, or at all. A significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. If we are unable to successfully increase the manufacturing capacity for a product candidate, the clinical trials as well as the regulatory approval or commercial launch of that product candidate may be delayed or there may be a shortage in supply. Our product candidates require precise, high-quality manufacturing in accordance with cGMP. Our failure to achieve and maintain these high-quality manufacturing standards in collaboration with our third-party manufacturers, including the incidence of manufacturing errors, could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could harm our business, financial condition and results of operations.
Our product candidates, if approved for sale, may not gain acceptance among physicians, patients, and the medical community, thereby limiting our potential to generate revenues.
If our product candidates are approved for commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product by physicians, healthcare professionals and third-party payors and our profitability and growth will depend on a number of factors, including:
| ● | demonstration of safety and efficacy; | |
| ● | perceived advantages of our product candidates over alternative treatments; | |
| ● | the indications for which the product candidates are approved and the labeling approved by regulatory authorities for use with the product candidates, including any warnings, limitations or contraindications contained in a product’s approved labeling; | |
| ● | approval of other new therapies for the same indications; | |
| ● | acceptance by physicians and patients of the product candidate as a safe and effective treatment; | |
| ● | the cost, safety and efficacy of treatment in relation to alternative treatments, including generic versions of the product candidates; | |
| ● | the extent to which our product candidates are included on formularies of hospitals and managed care organizations; | |
| ● | changes in the practice guidelines and the standard of care for the targeted indication; | |
| ● | relative convenience and ease of administration; | |
| ● | the prevalence and severity of any adverse side effects; | |
| ● | budget impact of adoption of our product on relevant drug formularies and the availability, cost, and potential advantages of alternative treatments, including less expensive generic drugs; | |
| ● | pricing, reimbursement, and cost effectiveness, which may be subject to regulatory control; | |
| ● | effectiveness of our or any of our partners’ sales and marketing strategies; | |
| ● | the product labeling or product insert required by the FDA or regulatory authority in other countries; and | |
| ● | the availability of adequate third-party insurance coverage or reimbursement. |
| 63 |
If any product candidate that we develop does not provide a treatment regimen that is as beneficial as, or is perceived as being as beneficial as, the current standard of care or otherwise does not provide patient benefit, that product candidate, if approved for commercial sale by the FDA or other regulatory authorities, likely will not achieve market acceptance. Our ability to effectively promote and sell any approved products will also depend on pricing and cost-effectiveness, including our ability to produce a product at a competitive price and our ability to obtain sufficient third-party coverage or reimbursement. If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, patients and third-party payors, our ability to generate revenues from that product would be substantially reduced. In addition, our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources, may be constrained by FDA rules and policies on product promotion, and may never be successful.
Guidelines and recommendations published by various organizations can impact the use of our products.
Government agencies promulgate regulations and guidelines directly applicable to us and to our product. In addition, professional societies, practice management groups, private health and science foundations and organizations involved in various diseases from time to time may also publish guidelines or recommendations to the healthcare and patient communities. Recommendations of government agencies or these other groups or organizations may relate to such matters as usage, dosage, route of administration and use of concomitant therapies. Recommendations or guidelines suggesting the reduced use of our products or the use of competitive or alternative products that are followed by patients and healthcare providers could result in decreased use of our proposed products.
The market for RenovoGem and our other product candidates may not be as large as we expect.
Our estimates of the potential market opportunity for RenovoGem and our other product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research reports and other surveys, including surveys commissioned by us. These assumptions include the size of our target populations, the prevalence and incidence of each of our target indications, the number of patients receiving current treatment, the percentage of patients unsatisfied with the current treatments, the number of diagnosed but untreated patients, the compliance and adherence of patients in our target populations, the number of treatment centers and prescribing physicians and the percentage of payer acceptance. While we believe that our internal assumptions are reasonable, if any of these assumptions proves to be inaccurate, then the actual market for our product candidates could be smaller than our estimates of our potential market opportunity. If the actual market for any of our product candidates is smaller than we expect, our product revenue may be limited, and it may be more difficult for us to achieve or maintain profitability.
In the event that we need to change our third-party contract manufacturers, our preclinical studies, clinical trials or the commercialization of our product candidates could be delayed, adversely affected or terminated, or such a change may result in significantly higher costs.
Due to regulatory restrictions inherent in an IND or NDA, or for economic reasons, various steps in the manufacture of any of our product candidates may need to be sole-sourced. We currently obtain our RenovoCath delivery system, subject to requirements under the QSR, from a single supplier. In accordance with cGMP regulations and QSR, changing manufacturers may require the re-validation of manufacturing processes and procedures, and may require further preclinical studies or clinical trials to show comparability between the materials produced by different manufacturers, and further regulatory review and approval. Changing our current or future contract manufacturers may be difficult for us and could be costly, which could result in our inability to manufacture our product candidate for an extended period of time and therefore a delay in the development of any of our product candidates. While we intend to find alternative suppliers to mitigate the risk, our efforts may not be successful. Further, to maintain our development timelines in the event of a change in our third-party contract manufacturer, we may incur significantly higher costs to manufacture any of our product candidates.
| 64 |
We currently do not have any internal drug discovery capabilities, and therefore we are dependent on identifying drugs that are off patent or on in-licensing or acquiring development programs from third parties in order to obtain additional product candidates.
If in the future we decide to further expand our pipeline of product candidates, we will be dependent on identifying drugs that are off patent or on in-licensing or acquiring product candidates as we do not have significant internal discovery capabilities at this time. We may face substantial competition from other biotechnology and pharmaceutical companies, many of which may have greater resources than we have, in obtaining in-licensing, sponsored research or acquisition opportunities. In-licensing or acquisition opportunities may not be available to us on terms we find acceptable, if at all. In-licensed compounds that appear promising in research or in preclinical studies may fail to progress into further preclinical studies or clinical trials.
If a product liability claim is successfully brought against us for uninsured liabilities, or such a claim exceeds our insurance coverage, we could be forced to pay substantial damage awards that could materially harm our business.
The use of any of our existing or future product candidates in clinical trials and the sale of any approved pharmaceutical products may expose us to significant product liability claims. We have product liability insurance coverage for our proposed clinical trials; however, such insurance coverage may be inadequate and may not protect us against any or all of the product liability claims that may be brought against us now or in the future. We may not be able to acquire or maintain adequate product liability insurance coverage at a commercially reasonable cost or in sufficient amounts or scope to protect us against potential losses. In the event a product liability claim is brought against us, we may be required to pay legal and other expenses to defend the claim, as well as uncovered damage awards resulting from a claim brought successfully against us. In the event that any of our product candidates are approved for sale by the FDA and commercialized, we may need to substantially increase the amount of our product liability coverage. Defending any product liability claim or claims could require us to expend significant financial and managerial resources, which could have a material adverse effect on our business.
We may delay or terminate the development of our product candidates at any time if we believe the perceived market or commercial opportunity does not justify further investment, which could materially harm our business.
Even though the results of preclinical studies and clinical trials that have been conducted or may be conducted in the future may support further development of our product candidates, we may delay, suspend or terminate the future development of a product candidate at any time for strategic, business, financial or other reasons, including the determination or belief that the emerging profile of the product candidate is such that it may not receive FDA approval, gain meaningful market acceptance, generate a significant return to stockholders, or otherwise provide any competitive advantages in its intended indication or market.
We may pursue a strategy of commercializing RenovoCath as a standalone device. If we pursue this strategy and are unable to generate revenues accordingly, our business would be harmed.
We may explore a strategy where, depending on a number of factors, we may consider selling RenovoCath as a standalone catheter (i.e., without any specific cancer therapeutic) for use by oncology surgeons. We expect to research this opportunity during 2024 by exploring the potential addressable market, regulatory requirements, sales and marketing strategies and the potential for insurance reimbursement. No assurances can be given that we will ultimately pursue this opportunity or, if we do, that we will be able achieve all of the prerequisites necessary for such commercialization or to generate any meaningful revenues from such commercialization. If we pursue this strategy and are unable to capitalize on it, we will suffer losses and our business and results of operations will be harmed.
| 65 |
Risks Related to Our Operations
Our future success depends on our ability to retain our key personnel and to attract, retain and motivate qualified personnel.
We are highly dependent on the development, regulatory, commercialization, and business development expertise of Shaun Bagai, our Chief Executive Officer, as well as the other principal members of our management, scientific and clinical teams. Although we have employment agreements, offer letters or consulting agreements with our executive officers, these agreements do not prevent them from terminating their services at any time.
If we lose one or more of our executive officers or key employees, our ability to implement our business strategy successfully could be seriously harmed. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop product candidates, gain regulatory approval, and commercialize new products. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain or motivate these additional key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition, including a recent hyper-competitive compensation environment, for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be engaged by entities other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. One such key consultant is Dr. Ramtin Agah, our Chief Medical Officer. If we are unable to continue to attract and retain highly qualified personnel, our ability to develop and commercialize product candidates will be limited.
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
We are a small company with less than 10 employees. The future growth of our company will impose significant additional responsibilities on members of management, including the need to identify, attract, retain, motivate and integrate highly skilled personnel. We may increase the number of employees in the future depending on the progress of our development and commercialization of our product candidates. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to:
| ● | manage our clinical studies effectively; | |
| ● | integrate additional and future management, administrative, manufacturing, sales and marketing, and regulatory personnel; | |
| ● | maintain sufficient administrative, accounting and management information systems and controls; and | |
| ● | hire and train additional qualified personnel. |
There is no guarantee that we will be able to accomplish these tasks, and our failure to accomplish any of them could materially adversely affect our business, prospects, and financial condition.
Business disruptions could seriously harm future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our third-party manufacturers, contract research organizations (“CROs”), and other contractors and consultants, could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions beyond our control, for which we are predominantly self-insured. The occurrence of any of these business disruptions could delay our clinical trials, seriously harm our operations and financial condition and increase our costs and expenses. In addition, our ability to obtain clinical supplies for our clinical trials and materials for our product candidates could be disrupted if the operations of these suppliers are affected by a man-made or natural disaster or other business interruptions.
Our corporate headquarters are located in Silicon Valley, California, an area prone to wildfires and earthquakes. These and other natural disasters could severely disrupt our operations, and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage or other event occurred that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as the manufacturing facilities of our third-party contract manufacturers, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. Any disaster recovery and business continuity plans we have in place may prove inadequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business.
| 66 |
Catastrophic events and man-made problems, such as terrorism, war, or climate change may disrupt our business.
A significant natural disaster, such as an earthquake, fire, flood, hurricane, or significant power outages, water shortages and the risks associated with climate change could have an adverse impact on our business, results of operations, and financial condition. Our employees and executive officers are located in the San Francisco Bay Area, a region known for seismic activity, drought, and wildfires, and the resultant air quality impacts and power outages associated with such wildfires.
In addition, acts of terrorism, public health emergencies, protests, riots, and the increasing frequency and impact of extreme weather events on critical infrastructure in the U.S. and elsewhere have the potential to disrupt our business and the business of our third-party suppliers, and may cause us to experience higher attrition, losses, and additional costs to maintain or resume operations. All of the aforementioned risks may be further increased if our course of action in response to catastrophic events proves to be inadequate. For example, if a catastrophic event occurred that prevented us from using all or a significant portion of our facility, that damaged critical infrastructure, such as the manufacturing facilities of our third-party contract manufacturers, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business for a substantial period of time. Any disaster recovery and business continuity plan we have in place may prove inadequate in the event of a serious disaster or similar event and we may incur substantial expenses as a result of the limited nature of these plans, which could have a material adverse effect on our business.
In February 2022, armed conflict escalated between Russia and Ukraine which led to various sanctions by the U.S. and other countries against Russia. Furthermore, ongoing war and instability in Israel and the surrounding region that commenced in October 2023 have added to global geopolitical unrest. It is not possible to predict the broader consequences of these conflicts, which could include further sanctions, embargoes, regional instability, prolonged periods of higher inflation, geopolitical shifts, and adverse effects on macroeconomic conditions, currency exchange rates, and financial markets, all of which could have a material adverse effect on our business, financial condition, and results of operations.
Security threats to our information technology infrastructure and/or our physical buildings could expose us to liability and damage our reputation and business.
It is essential to our business strategy that our and our vendors, partners, clinical trial sites, and third-party providers’ technology and network infrastructure and physical buildings remain secure and are perceived by our customers and corporate partners to be secure. Despite our implementation of security measures, any of the internal computer systems and networks belonging to or used by us or our employees and our third-party service providers are vulnerable to damage and disruption from computer viruses, ransomware and other malicious code, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failure, as well as security breaches and incidents from inadvertent or intentional actions, or from cyber-attacks by malicious third parties (including supply chain cyber-attacks, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information), which may compromise system infrastructure or lead to the loss, destruction, alteration, prevention of access to, disclosure, or dissemination of, or damage or unauthorized access to, our data (including trade secrets or other confidential information, intellectual property, proprietary business information, and personal information) or data that is processed or maintained on our behalf, or other assets, which could result in financial, legal, business and reputational harm to us. Any system failure, accident or security breach or incident that causes interruptions in our own or in our third-party service providers’ operations could result in a material disruption of our development programs or other aspects of our operations. As a result of the advent of remote working, with many of our employees working from home from time to time and accessing our corporate network via remote devices, the potential for such events to occur is even greater. Despite security measures, we also cannot guarantee the security of our physical buildings. Physical building penetration or any cyber-attacks could negatively affect our reputation, damage our network infrastructure and our ability to deploy our products and services, harm our relationship with customers and partners that are affected, and expose us to financial liability, including the possibility of consequential damages resulting from cyber-attacks and other security threats.
| 67 |
Additionally, there are a number of state, federal, and international laws protecting the privacy and security of health information and personal data. For example, the HIPAA imposes limitations on the use and disclosure of an individual’s healthcare information by healthcare providers, healthcare clearinghouses, and health insurance plans, or, collectively, covered entities, and also grants individuals rights with respect to their health information. HIPAA also imposes compliance obligations and corresponding penalties for non-compliance on individuals and entities that provide services to healthcare providers and other covered entities. As part of the American Recovery and Reinvestment Act of 2009 (“ARRA”), the privacy and security provisions of HIPAA were amended. ARRA also made significant increases in the penalties for improper use or disclosure of an individual’s health information under HIPAA and extended enforcement authority to state attorneys general. As amended by ARRA and subsequently by the final omnibus rule adopted in 2013, HIPAA also imposes notification requirements on covered entities in the event that certain health information has been inappropriately accessed or disclosed, including notification requirements to individuals, federal regulators, and in some cases, notification to local and national media. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with encryption or other standards developed by the U.S. Department of Health and Human Services. Most states have laws requiring notification of affected individuals and/or state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms, to ensure ongoing protection of personal information. Activities outside of the U.S. implicate local and national data protection standards, impose additional compliance requirements, and generate additional risks of enforcement for non-compliance. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws, to protect against security breaches and hackers or to alleviate problems caused by such breaches.
We and our third-party contract manufacturers must comply with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant costs or liabilities.
We and our third-party manufacturers are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the use, generation, manufacture, distribution, storage, handling, treatment, remediation and disposal of hazardous materials and wastes. Hazardous chemicals, including flammable and biological materials, are involved in certain aspects of our business, and we cannot eliminate the risk of injury or contamination from the use, generation, manufacture, distribution, storage, handling, treatment or disposal of hazardous materials and wastes. In the event of contamination or injury, or failure to comply with environmental, health and safety laws and regulations, we could be held liable for any resulting damages and any such liability could exceed our assets and resources. We could also incur significant costs associated with civil or criminal fines and penalties for failure to comply with such laws and regulations.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
Environmental, health and safety laws and regulations are becoming increasingly more stringent. We may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Further, with respect to the operations of our third-party contract manufacturers, it is possible that if they fail to operate in compliance with applicable environmental, health and safety laws and regulations or properly dispose of wastes associated with our products, we could be held liable for any resulting damages, suffer reputational harm or experience a disruption in the manufacture and supply of our product candidates or products.
| 68 |
A variety of risks associated with operating internationally could materially adversely affect our business.
Doing business internationally involves a number of risks, including but not limited to:
| ● | multiple, conflicting and changing laws and regulations, such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses; | |
| ● | failure by us to obtain and maintain regulatory approvals for the use of our products in various countries; | |
| ● | additional potentially relevant third-party patent rights; | |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; | |
| ● | difficulties in staffing and managing foreign operations; | |
| ● | complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems; | |
| ● | limits in our ability to penetrate international markets; | |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products, exposure to foreign currency exchange rate fluctuations, and a rising rate of inflation; | |
| ● | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; | |
| ● | certain expenses including, among others, expenses for travel, translation, and insurance; and | |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, its books and records provisions, or its anti-bribery provisions. |
Any of these factors could significantly harm any current or future international operations and, consequently, our results of operations.
General economic or business conditions may have a negative impact on our business.
Continuing concerns over U.S. healthcare reform legislation and energy costs, geopolitical issues, fluctuations in inflation rates, market volatility, the availability and cost of credit and government stimulus programs in the U.S. and other countries, as well as recent and potential future disruptions in access to bank deposits or lending commitments due to bank failure, have contributed to increased volatility and could materially and adversely affect our liquidity, our business and financial condition. The 2023 closures of Silicon Valley Bank and Signature Bank and their placement into receivership with the Federal Deposit Insurance Corporation (“FDIC”) created bank-specific and broader financial institution liquidity risk and concerns. Although the Department of the Treasury, the Federal Reserve, and the FDIC jointly released a statement that depositors at Silicon Valley Bank and Signature Bank would have access to their funds, even those in excess of the standard FDIC insurance limits, future adverse developments with respect to specific financial institutions or the broader financial services industry may lead to market-wide liquidity shortages. The failure of any bank in which we deposit our funds could reduce the amount of cash we have available for our operations or delay our ability to access such funds. Any such failure may increase the possibility of a sustained deterioration of financial market liquidity, or illiquidity at clearing, cash management and/or custodial financial institutions. In the event we have a commercial relationship with a bank that has failed or is otherwise distressed, we may experience delays or other issues in meeting our financial obligations. If other banks and financial institutions enter receivership or become insolvent in the future in response to financial conditions affecting the banking system and financial markets, our ability to access our cash and cash equivalents and investments may be threatened and could have a material adverse effect on our business and financial condition.
| 69 |
If the economic climate deteriorates or is poor, our business, as well as the financial condition of our suppliers and our third-party payors, could be negatively impacted, which could materially adversely affect our business, prospects and financial condition.
Our operations are subject to the effects of a rising rate of inflation.
The United States has recently experienced historically high levels of inflation. If the inflation rate continues to increase, for example due to increases in the costs of labor and supplies, it will affect our expenses, such as employee compensation and research and development charges. Additionally, the United States is experiencing an acute workforce shortage, which in turn, has created a very competitive wage environment that may increase the Company’s operating costs. To the extent inflation results in rising interest rates and has other adverse effects on the market, it may adversely affect our financial condition and results of operations.
Healthcare reform measures could adversely affect our business. The impact of recent healthcare reform legislation and other changes in the healthcare industry and in healthcare spending on us is currently unknown and may adversely affect our business model.
Existing regulatory policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
In the United States and foreign jurisdictions, there have been, and continue to be, a number of legislative and regulatory changes and proposed changes to the healthcare system that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the federal and state levels in the United States that seek to reduce healthcare costs. In 2010, the Patient Protection and Affordable Care Act (the “PPACA”) was enacted, which includes measures to significantly change the way healthcare is financed by both governmental and private insurers. Among the provisions of the PPACA of greatest importance to the pharmaceutical and biotechnology industry are the following:
| ● | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs; |
| ● | implementation of the federal physician payment transparency requirements, sometimes referred to as the “Physician Payments Sunshine Act”; | |
| ● | a licensure framework for follow-on biologic products; | |
| ● | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; | |
| ● | establishment of a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending; | |
| ● | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program, to 23.1% and 13% of the average manufacturer price for most branded and generic drugs, respectively and capped the total rebate amount for innovator drugs at 100% of the AMP; | |
| ● | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for certain drugs and biologics, including our product candidates, that are inhaled, infused, instilled, implanted or injected; |
| 70 |
| ● | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; | |
| ● | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for individuals with income at or below 133% of the federal poverty level, thereby potentially increasing manufacturers’ Medicaid rebate liability; | |
| ● | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; and | |
| ● | expansion of the entities eligible for discounts under the Public Health program. |
Since its enactment, there remain judicial and Congressional challenges to certain aspects of the PPACA. For example, in June 2021 the U.S. Supreme Court held that Texas and other challengers had no legal standing to challenge the PPACA, dismissing the case on procedural grounds without specifically ruling on the constitutionality of the PPACA. Thus, the PPACA will remain in effect in its current form. In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 due to subsequent legislative amendments will remain in effect through 2032, with the exception of a temporary suspension implemented under various COVID-19 relief legislation.
Moreover, there has recently been heightened governmental scrutiny over the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. For example, under the American Rescue Plan Act of 2021, effective January 1, 2024, the statutory cap on Medicaid Drug Rebate Program rebates that manufacturers pay to state Medicaid programs will be eliminated. Elimination of this cap may require pharmaceutical manufacturers to pay more in rebates than it receives on the sale of products, which could have a material impact on our business. In August 2022, Congress passed the Inflation Reduction Act of 2022, which includes prescription drug provisions that have significant implications for the pharmaceutical industry and Medicare beneficiaries, including allowing the federal government to negotiate a maximum fair price for certain high-priced single source Medicare drugs, imposing penalties and excise tax for manufacturers that fail to comply with the drug price negotiation requirements, requiring inflation rebates for all Medicare Part B and Part D drugs, with limited exceptions, if their drug prices increase faster than inflation, and redesigning Medicare Part D to reduce out-of-pocket prescription drug costs for beneficiaries, among other changes. Various industry stakeholders, including certain pharmaceutical companies and industry interest groups, have initiated lawsuits against the federal government asserting that the price negotiation provisions of the Inflation Reduction Act are unconstitutional. The impact of these judicial challenges and future legislative, executive, and administrative actions and any agency rules on us and the pharmaceutical industry as a whole is unclear. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our product candidates if approved. Complying with any new legislation and regulatory changes could be time-intensive and expensive, resulting in a material adverse effect on our business.
Individual states have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm our business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for our products or put pressure on our product pricing, which could negatively affect our business, results of operations, financial condition and prospects.
| 71 |
In addition, given recent federal and state government initiatives directed at lowering the total cost of healthcare, Congress and state legislatures will likely continue to focus on healthcare reform, the cost of prescription drugs and biologics and the reform of the Medicare and Medicaid programs. While we cannot predict the full outcome of any such legislation, it may result in decreased reimbursement for drugs and biologics, which may further exacerbate industry-wide pressure to reduce prescription drug prices. This could harm our ability to generate revenue. Further, a number of states are considering or have recently enacted state drug price transparency and reporting laws that could substantially increase our compliance burdens and expose us to greater liability under such state laws once we begin commercialization.
Increases in importation or re-importation of pharmaceutical products from foreign countries into the United States could put competitive pressure on our ability to profitably price our products, which, in turn, could adversely affect our business, results of operations, financial condition and prospects. We might elect not to seek approval for or market our products in foreign jurisdictions in order to minimize the risk of re-importation, which could also reduce the revenue we generate from product sales. It is also possible that other legislative proposals having similar effects will be adopted.
Furthermore, regulatory authorities’ assessment of the data and results required to demonstrate safety and efficacy can change over time and can be affected by many factors, such as the emergence of new information, including on other products, changing policies and agency funding, staffing and leadership. We cannot be sure whether future changes to the regulatory environment will be favorable or unfavorable to our business prospects. For example, average review times at the FDA for marketing approval applications can be affected by a variety of factors, including budget and funding levels and statutory, regulatory and policy changes.
Reimbursement for any approved products may be limited or unavailable, which could make it difficult for us to sell our product candidates profitably.
In both domestic and foreign markets, sales of any of our other product candidates, if approved, will depend, in part, on the extent to which the costs of our product candidates will be covered by third-party payors, such as government health care programs, commercial insurance and managed health care organizations. These third-party payors decide which drugs will be covered and establish reimbursement levels for those drugs. The containment of health care costs has become a priority of foreign and domestic governments as well as private third-party payors. The prices of drugs have been a focus in this effort. Governments and private third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications, which could affect our ability to sell our product candidates profitably. Cost-control initiatives could cause us to decrease the price we might establish for product candidates, which could result in lower than anticipated product revenues.
Reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a product is:
| ● | a covered benefit under its health plan; | |
| ● | safe, effective and medically necessary; | |
| ● | appropriate for the specific patient; | |
| ● | cost-effective relative to other alternatives, including generic products; and | |
| ● | neither experimental nor investigational. |
Adverse pricing limitations may hinder our ability to recoup our investment in our existing and any future product candidates, even if such product candidates obtain marketing approval.
| 72 |
Obtaining coverage and reimbursement approval for a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of our product candidates to the payor. Further, there is significant uncertainty related to third-party payor coverage and reimbursement of newly approved product candidates, including our product candidates if they are approved. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. We cannot be sure that coverage or adequate reimbursement will be available for any of our product candidates. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our product candidates. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize certain of our product candidates. In addition, in the United States, third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new product candidates. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for their use of newly approved product candidates, which in turn will put pressure on pricing.
In some countries, including member states of the European Union, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of marketing approval for a product. In addition, there can be considerable pressure from governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member states and other countries and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our product candidates is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected.
Any legal proceedings or claims against us could be costly and time-consuming to defend and could harm our reputation regardless of the outcome.
We may in the future become subject to legal proceedings and claims that arise in the ordinary course of business, including intellectual property, product liability, employment, wage and hour, class action, derivative, whistleblower and other litigation claims, and governmental and other regulatory investigations and proceedings. Such matters can be time-consuming, divert management’s attention and resources, cause us to incur significant expenses or liability, or require us to change our business practices. In addition, the expense of litigation, for which we are either not insured or only partially insured depending on the claim, and the timing of this expense from period to period will be difficult to estimate, subject to change, and could adversely affect our financial condition and results of operations. Because of the potential risks, expenses, and uncertainties of litigation, we may, from time to time, settle disputes, even where we have meritorious claims or defenses, by agreeing to settlement agreements. Any of the foregoing could adversely affect our business, financial condition, and results of operations.
Changes in tax laws or in their implementation or interpretation may adversely affect our business and financial condition
We are or may become subject to income and non-income taxes in the United States under federal, state and local jurisdictions and in certain foreign jurisdictions in which we operate. Tax laws, regulations and administrative practices in these jurisdictions may be subject to significant change, with or without advance notice. For example, on January 1, 2022, a provision of the Tax Cuts and Jobs Act of 2017 went into effect that eliminates the option to deduct domestic research and development costs in the year incurred and instead requires taxpayers to amortize such costs over five years. The Company is currently evaluating the potential impact. Also, the Inflation Reduction Act, which introduced a 15% minimum tax on book income and a 1% excise tax on stock buybacks. Changes in tax laws (including provisions of the recently enacted federal tax legislation titled the Inflation Reduction Act), regulations, or rulings, changes in interpretations of existing laws and regulations, or changes in accounting principles could negatively and materially affect our financial position, effective tax rates, cash flows, and results of operations.
| 73 |
Risks Related to Intellectual Property
If we are unable to protect our intellectual property effectively, we may be unable to prevent third parties from using our technologies, which would impair our competitive advantage.
We rely on patent protection as well as a combination of trademark, copyright and trade secret protection, and other contractual restrictions, to protect our proprietary technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. We may not be successful in defending challenges made in connection with our patents and patent applications. If we fail to protect our intellectual property, we will be unable to prevent third parties from using our technologies and they will be able to compete more effectively against us.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example, others may be able to make products that are similar to our product candidates or utilize similar technology but that are not covered by the claims of the patents that we license or may own; we, or our future licensors or collaborators, might not have been the first to file patent applications covering certain of our or their inventions; and issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors or other third parties.
In addition to our patents, we rely on contractual restrictions to protect our proprietary technology. We require our employees and third parties to sign confidentiality agreements and our employees are also required to sign agreements assigning to us all intellectual property arising from their work for us. Nevertheless, we cannot guarantee that these measures will be effective in protecting our intellectual property rights. Should any of these events occur, it or they could have a material adverse effect on our business, financial condition, results of operations, and growth prospects.
If any of our patent applications do not issue as patents in any jurisdiction, we may not be able to compete effectively.
Our currently pending or future patent applications may not result in issued patents and any patents issued to us may be challenged, invalidated, or held unenforceable. Changes in either the patent laws or their interpretation in the U.S. and other countries may diminish our ability to protect our inventions, obtain, maintain, and enforce our intellectual property rights, and, more generally, could affect the value of our intellectual property or narrow the scope of our patents with respect to our product candidates. Furthermore, we cannot be certain that we were the first to make the invention claimed in our issued patents or pending patent applications in the U.S., or that we were the first to file for protection of the inventions claimed in our foreign issued patents or pending patent applications.
There are numerous recent changes to the patent laws and proposed changes to the rules of the United States Patent and Trademark Office (“USPTO”), which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For example, in September 2011, the U.S. enacted sweeping changes to the U.S. patent system under the Leahy-Smith America Invents Act, including changes that transitioned the U.S. from a “first-to-invent” system to a “first-to-file” system and alter the processes for challenging issued patents. These changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. In addition, we may become subject to interference proceedings conducted in the patent and trademark offices of various countries to determine our entitlement to patents, and these proceedings may conclude that other patents or patent applications have priority over our patents or patent applications.
It is also possible that a competitor may successfully challenge our patents through various proceedings and those challenges may result in the elimination or narrowing of our patents, and therefore reduce our patent protection. The patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output in time to obtain patent protection. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed to third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. Any parties who enter into nondisclosure or confidentiality agreements with us that have access to confidential or patentable aspects of our research and development output may breach such agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection. In addition, rights under any of our issued patents, patent applications or future patents may not provide us with commercially meaningful protection for our products or afford us a commercial advantage against our competitors or their competitive products or processes.
| 74 |
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our unregistered trademarks or trade names may be challenged, infringed, circumvented, or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our unregistered trademarks or trade names. Over the long term, if we are unable to successfully register our trademarks and trade names and establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely impact our financial condition or results of operations.
The patents issued to us may not be broad enough to provide any meaningful protection, one or more of our competitors may develop more effective technologies, designs or methods without infringing our intellectual property rights and one or more of our competitors may design around our proprietary technologies.
If we are not able to protect our proprietary technology, trade secrets and know-how, our competitors may use our inventions to develop competing products. Our patents may not protect us against our competitors, and patent litigation is very expensive. We may not have sufficient cash available to pursue any patent litigation to its conclusion because we currently do not generate revenues other than licensing, milestone and royalty income.
We cannot rely solely on our current patents to be successful. The standards that the USPTO and foreign patent office’s use to grant patents, and the standards that U.S. and foreign courts use to interpret patents, are not the same, are not always applied predictably or uniformly and can change, particularly as new technologies develop. As such, the degree of patent protection obtained in the U.S. may differ substantially from that obtained in various foreign countries.
We cannot be certain of the level of protection, if any, that will be provided by our patents if they are challenged in court, where our competitors may raise defenses such as invalidity, unenforceability, or possession of a valid license. In addition, the type and extent of any patent claims that may be issued to us in the future are uncertain. Any patents that are issued may not contain claims that will permit us to stop competitors from using similar technology.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights.
Third parties may challenge the validity, inventorship or ownership of our patents and other intellectual property rights, resulting in costly litigation or other time-consuming and expensive proceedings, which could deprive us of valuable rights. If we become involved in any intellectual property litigation, interference or other judicial or administrative proceedings, we will incur substantial expenses and the attention of our technical and management personnel will be diverted. An adverse determination may subject us to significant liabilities or require us to seek licenses that may not be available from third parties on commercially favorable terms, if at all. Further, if such claims are proven valid, through litigation or otherwise, we may be required under applicable law to pay substantial monetary damages, which can be tripled if the infringement is deemed willful, or be required to discontinue or significantly delay development, marketing, selling and licensing of the affected products and intellectual property rights.
Our competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application may have priority over our patent applications and could further require us to obtain rights to issued patents covering such technologies. There may be third-party patents, patent applications and other intellectual property relevant to our potential products that may block or compete with our potential products or processes. If another party has filed a U.S. patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the USPTO to determine priority of invention in the U.S. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in a loss of our U.S. patent position with respect to such inventions. In addition, we cannot assure you that we would prevail in any of these suits or that the damages or other remedies that we are ordered to pay, if any, would not be substantial. Claims of intellectual property infringement, misappropriation or other violations against us may require us to enter into royalty or license agreements with third parties that may not be available on acceptable terms, if at all. We may also be subject to injunctions against the further development and use of our technology, which could materially adversely affect our business, prospects and financial condition.
| 75 |
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could materially adversely affect our ability to raise the funds necessary to continue our operations.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. In the United States, patents have a limited lifespan, and if all maintenance fees are timely paid, the natural expiration of a patent is generally 20-years from its earliest U.S. non-provisional filing date. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent application process and following the issuance of a patent. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In certain circumstances, even inadvertent noncompliance events may permanently and irrevocably jeopardize patent rights. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
If we are unable to obtain licenses from third parties on commercially reasonable terms or fail to comply with our obligations under such agreements, our business could be harmed.
It may be necessary for us to use the patented or proprietary technology of third parties to commercialize our products, in which case we would be required to obtain a license from these third parties. If we are unable to license such technology, or if we are forced to license such technology, on unfavorable terms, our business could be materially harmed. If we are unable to obtain a necessary license, we may be unable to develop or commercialize the affected product candidates, which could materially harm our business, and the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation. Even if we are able to obtain a license, agreements we may enter into in the future, if any, may not provide exclusive rights to use certain intellectual property and technology retained by the collaborator in all relevant fields of use and in all territories in which we may wish to develop or commercialize our technology and products in the future. As a result, we may not be able to prevent competitors or other third parties from developing and commercializing competitive products that utilize technology retained by such collaborators to the extent such products are not also covered by our intellectual property. In such an event, our business, financial condition, results of operations, and growth prospects could be materially harmed.
We rely on confidentiality agreements to protect our trade secrets. If these agreements are breached by our employees or other parties, our trade secrets may become known to our competitors. We may also be subject to claims that our employees, consultants, or advisors have wrongfully used or disclosed alleged trade secrets or claims asserting ownership of what we regard as our own intellectual property.
We rely on trade secrets that we seek to protect through confidentiality agreements with our employees and other parties. If these agreements are breached, our competitors may obtain and use our trade secrets to gain a competitive advantage over us. We may not have any remedies against our competitors and any remedies that may be available to us may not be adequate to protect our business or compensate us for the damaging disclosure. In addition, we may have to expend resources to protect our interests from possible infringement by others.
| 76 |
In addition, although we try to ensure that our employees, consultants, and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these individuals have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims.
Risks Related to Our Common Stock
An active trading market for our common stock may not be sustained.
Prior to the closing of our IPO in August 2021, there was no public trading market for our common stock. Although our common stock is listed on the Nasdaq Capital Market, the market for our shares has demonstrated varying levels of trading activity. Our ability to raise capital to continue to fund operations by selling shares of our common stock and our ability to acquire other companies or technologies by using shares of our common stock as consideration may be impaired if an active trading market for our common stock is not sustained.
The market price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for investors.
The market price of our common stock is likely to be highly volatile and may be subject to wide fluctuations in response to a variety of factors, some of which are beyond our control. These factors include the following:
| ● | any delay in the commencement, enrollment and ultimate completion of our clinical trials; | |
| ● | any delay in submitting an NDA and any adverse development or perceived adverse development with respect to the FDA’s review of that NDA; | |
| ● | failure to successfully develop and commercialize RenovoGem; | |
| ● | inability to obtain additional funding; | |
| ● | regulatory or legal developments in the United States and other countries applicable to RenovoGem or any other product candidate; | |
| ● | adverse regulatory decisions; | |
| ● | changes in the structure of healthcare payment systems; | |
| ● | inability to obtain adequate product supply for RenovoGem, RenovoCath or any other product candidate, or the inability to do so at acceptable prices; | |
| ● | introduction of new products, services or technologies by our competitors; | |
| ● | failure to meet or exceed financial projections we provide to the public; | |
| ● | failure to meet or exceed the estimates and projections of the investment community; | |
| ● | changes in the market valuations of companies similar to ours; | |
| ● | market conditions in the pharmaceutical and biotechnology sectors, and the issuance of new or changed securities analysts’ reports or recommendations; | |
| ● | announcements of significant acquisitions, strategic collaborations, joint ventures or capital commitments by us or our competitors; |
| 77 |
| ● | significant lawsuits, including patent or stockholder litigation, and disputes or other developments relating to our proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; | |
| ● | additions or departures of key scientific or management personnel; | |
| ● | sales of our common stock or other securities by us, our insiders or our other stockholders, including pursuant to the existing primary and secondary shelf registration statements that we have filed with the SEC; | |
| ● | expiration of market standoff or lock-up agreements; | |
| ● | trading volume of our common stock; | |
| ● | fluctuations in interest rates and inflation rates; | |
| ● | general economic, industry and market conditions; | |
| ● | health epidemics and outbreaks or other natural or man-made disasters which could significantly disrupt our preclinical studies and clinical trials, and therefore our receipt of necessary regulatory approvals could be delayed or prevented; and | |
| ● | the other factors described in this “Risk Factors” section. |
In addition, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. This is particularly true for biotechnology companies like ours. These fluctuations have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors, as well as general economic, political, regulatory and market conditions, may negatively affect the market price of our common stock, regardless of our actual operating performance.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our product candidates on unfavorable terms to us.
We have and will likely continue to seek additional capital through a variety of means, including through public or private equity, debt financings or other sources, including up-front payments and milestone payments from strategic collaborations. For example, we have filed an omnibus shelf registration statement on Form S-3 that provides for aggregate offerings of up to $50 million of our securities subject to various limitations, including limited sales in any twelve-month period while we are subject to the “baby-shelf” rules. To the extent that we raise additional capital through the sale of equity or convertible debt or equity securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Such financing may result in dilution to stockholders, imposition of debt covenants, increased fixed payment obligations or other restrictions that may affect our business. If we raise additional funds through up-front payments or milestone payments pursuant to strategic collaborations with third parties, we may have to relinquish valuable rights to our product candidates or grant licenses on terms that are not favorable to us. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
If we fail to regain compliance with or meet all applicable Nasdaq requirements, we could be delisted from Nasdaq, which would seriously harm the liquidity of our stock and our ability to raise capital.
Our common stock is listed on the Nasdaq Capital Market. In order to maintain our listing, we must meet minimum financial and other requirements, including a minimum amount in stockholders’ equity.
On August 21, 2023, we received a notice from Nasdaq notifying us that, as of August 18, 2023, the Company was not in compliance with the minimum stockholders’ equity requirement for continued listing on The Nasdaq Capital Market, under Listing Rule 5550(b)(1), because the Company’s stockholders’ equity of $1,188,000 as reported in the Company’s Quarterly Report on Form 10-Q for the period ended June 30, 2023 was below the required minimum of $2.5 million (the “Equity Requirement”), and because, as of June 30, 2023, we did not meet the alternative compliance standards, relating to the market value of listed securities of $35 million or net income from continuing operations of $500,000 in the most recently completed fiscal year.
| 78 |
In accordance with the Nasdaq Listing Rules 5810(c)(2)(C), we were provided 45 calendar days from August 21, 2023, or until October 5, 2023, to submit a plan to regain compliance with the Equity Requirement (the “Compliance Plan”). We submitted the Compliance Plan to Nasdaq on October 5, 2023. On October 31, 2023, we received formal notice that the Staff had granted our request for continued listing on Nasdaq pursuant to an extension, ultimately through February 19, 2024, to evidence full compliance with all applicable criteria for continued listing on The Nasdaq Capital Market, including the $2.5 million stockholders’ equity requirement set forth in Nasdaq Listing Rule 5550(b)(2).
On February 21, 2024, we received a from Nasdaq that we had failed to meet the Minimum Stockholders’ Equity Requirement and that Nasdaq would commence delisting proceedings against us unless we timely requested a hearing before the Nasdaq Hearing Panel (the “Hearing Panel”). On February 28, 2024, we filed a request to appeal any delisting or suspension action by the Nasdaq staff at least pending the issuance of the Hearing Panel’s decision and the expiration of any extension that may be granted to us following the hearing. The hearing is scheduled on April 23, 2024.
If are unable to convince the Hearing Panel to afford us extra time to satisfy the Nasdaq’s listing requirements, or if we are afforded such time and thereafter fail to meet such requirement, our common stock would be subject to delisting by Nasdaq. If the Nasdaq delists our common stock, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; | |
| ● | a determination that our common stock is a “penny stock” which will require brokers trading in our common stock to adhere to more stringent rules and possibly resulting in a reduced level of trading activity in the secondary trading market for our common stock; | |
| ● | a limited amount of news and analyst coverage for our Company; and | |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
Sales of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur, including shares of common stock issued in this our January 2024 private placement, could depress the market price of our common stock.
Sales of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur as a result of our utilization of a universal shelf registration statement or otherwise could depress the market price of our common stock and impair our ability to raise capital through the sale of additional equity securities. Notably, we are required to register for public resale in the very near term all of the shares of common stock and common stock underlying warrants issued in our January 2024 private placement, which collectively will represent a very large number of shares relative to our current shares of common stock outstanding. Following the effectiveness of such registration, a large number of shares of our common stock could be sold in the public market, depressing our stock price. Moreover, we cannot in general predict the effect that future sales of our common stock or the market perception that we are permitted to sell a significant number of our securities would have on the market price of our common stock.
We could be subject to securities class action litigation.
In the past, securities class action and derivative litigation has often been brought against companies following a decline in the market price of their securities or upon the occurrence of other corporate events. This risk is especially relevant for us because biotechnology companies have experienced significant share price volatility in recent years. If we face such litigation, it could result in substantial costs, for which we are not insured, and a diversion of management’s attention and resources, which could harm our business.
| 79 |
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the market price for the shares and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us or our business. If our revenue or operating results fall below the expectations of analysts or investors or below any forecasts we may provide to the market, or if the forecasts we provide to the market are below the expectations of analysts or investors, the price of our common stock could decline substantially. If one or more of the analysts who cover us downgrades our common stock or publishes inaccurate or unfavorable research about our business, the market price for our common stock would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for our common stock to decline.
We do not expect to pay dividends in the foreseeable future, and you must rely on price appreciation of your shares for return on your investment.
We have paid no cash dividends on any class of our stock to date and we do not anticipate paying cash dividends in the near term. For the foreseeable future, we intend to retain any earnings to finance the development and expansion of our business, and we do not anticipate paying any cash dividends on our stock. Accordingly, investors must be prepared to rely on sales of their shares of common stock after price appreciation to earn an investment return, which may never occur. Investors seeking cash dividends should not purchase our shares of common stock. Any determination to pay dividends in the future will be made at the discretion of our board of directors and will depend on our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board deems relevant.
We have incurred and will continue to incur increased costs as a result of operating as a public company, and our management has devoted and will continue to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, we have incurred and particularly after we no longer qualify as an emerging growth company, we will continue to incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002 (“SOX”), the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of Nasdaq, and other applicable securities rules and regulations impose various requirements on U.S. reporting public companies, including the establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to continue to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have increased our legal and financial compliance costs and have made some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management personnel or members for our board of directors. In addition, these rules and regulations are often subject to varying interpretations, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of SOX (“Section 404”), we will be required to furnish a report by our senior management on our internal control over financial reporting beginning with our second filing of an Annual Report on Form 10-K with the SEC.
However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To prepare for eventual compliance with Section 404, once we no longer qualify as an emerging growth company, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404.
| 80 |
We have identified material weaknesses in our internal control over financial reporting. Failure to maintain effective internal controls could cause our investors to lose confidence in us and adversely affect the market price of our common stock. If our internal controls are not effective, we may not be able to accurately report our financial results or prevent fraud.
Effective internal control over financial reporting is necessary for us to provide reliable financial reports in a timely manner. In connection with the audit of our financial statements as of and for the years ended December 31, 2023, and 2022, we identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. Specifically, we determined that we lacked a sufficient number of qualified accounting and financial reporting personnel with an appropriate level of knowledge, training and experience to address complex accounting issues, sufficient written policies and procedures for accounting and financial reporting in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”), and adequate management review controls. In addition, we determined that our financial statement close process includes significant control gaps mainly driven by the small size of our accounting and finance staff and, as a result, a significant lack of appropriate segregation of duties. This includes the ability of users to create and post journal entries without adequate compensating review controls as well as review of system rights on the journal entry and financial close process. In addition, we did not have proper information technology general controls (ITGCs) related to user access, including the performance of user access reviews, access to edit data in applications was not properly restricted, and formal approval of application access was not documented and retained.
The above material weaknesses could result in a misstatement of our account balances or disclosures that would result in a material misstatement of our annual or interim financial statements that would not be prevented or detected. To address the material weaknesses, we have implemented, and are continuing to implement, measures designed to improve internal control over financial reporting, including expanding our accounting and finance team to add additional qualified accounting and finance resources, which may include third party consultants, and new financial processes. We intend to continue to take steps to remediate the material weaknesses through the hiring or engagement of additional experienced accounting and financial reporting personnel, formalizing documentation of policies and procedures and further evolving the accounting processes, including implementing appropriate segregation of duties. We expect to incur additional costs to remediate these weaknesses, including personnel, consulting and other costs.
We may not be effective in implementing these changes or in developing other internal controls, which may undermine our ability to provide accurate, timely and reliable reports on our financial and operating results. Further, we will not be able to fully assess whether the steps we are taking will remediate the material weakness in our internal control over financial reporting until we have completed our implementation efforts and sufficient time passes in order to evaluate their effectiveness. In addition, until we remediate these weaknesses, or if we identify additional material weaknesses in our internal control over financial reporting, we may not detect errors on a timely basis and our financial statements may be materially misstated. Moreover, in the future we may engage in business transactions, such as acquisitions, reorganizations or implementation of new information systems that could negatively affect our internal control over financial reporting and result in material weaknesses.
If we identify new material weaknesses in our internal control over financial reporting, if we are unable to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we are unable to assert that our internal control over financial reporting is effective, we may be late with the filing of our periodic reports, investors may lose confidence in the accuracy and completeness of our financial reports, and the market price of our common stock could be negatively affected. As a result of such failures, we could also become subject to investigations by the stock exchange on which our securities are listed, the SEC, or other regulatory authorities, and become subject to litigation from investors and stockholders, which could harm our reputation, financial condition or divert financial and management resources from our core business.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
As a public company, we maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated, communicated and discussed with our management, including our Chief Executive Officer and Principal Accounting Officer or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the desired control objectives will be met.
| 81 |
In reaching a reasonable level of assurance, management has weighed the cost of contemplated controls against their intended benefits. The design of any system of controls is based on management’s assumptions about the likelihood of future events. We cannot assure you that our controls will achieve their stated goals under all possible conditions. Changes in future conditions may render our controls inadequate or may cause our degree of compliance with them to deteriorate. These inherent limitations include the fact that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Principal Accounting Officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the year ended December 31, 2023. Based on this evaluation, our Chief Executive Officer and Principal Accounting Officer have concluded that, during the period covered by this Report, our disclosure controls and procedures were not effective due to our previously identified material weaknesses in internal control over financial reporting. As a result, we have performed additional analysis as deemed necessary to ensure that our financial statements were prepared in accordance with U.S. GAAP. Accordingly, notwithstanding the identified material weaknesses, management, including our Chief Executive Officer and Principal Accounting Officer, believes the financial statements included in this Report are fairly presented, in all material respects, in accordance with U.S. GAAP.
We are an “emerging growth company,” and the reduced reporting requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act (“JOBS Act”). For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including exemption from compliance with the auditor attestation requirements of Section 404; the ability to delay the implementation of new or revised financial accounting standards; reduced disclosure obligations regarding executive compensation; and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, the information we provide stockholders will be different than the information that is available with respect to other public companies. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the closing of our IPO, (b) in which we have total annual gross revenue of at least $1.235 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock held by non-affiliates exceeds $700 million as of the end of our prior second fiscal quarter, or (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
In addition, under the JOBS Act, emerging growth companies may delay adopting new or revised accounting standards until such time as those standards apply to private companies. We may elect not to avail ourselves of this exemption from new or revised accounting standards and, therefore, may be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result of these exemptions, there may be a less active trading market for our common stock and our share price may be more volatile.
| 82 |
Anti-takeover and other provisions contained in our certificate of incorporation and bylaws, as well as provisions of Delaware law, could impair a takeover attempt and have other impacts on our corporate governance.
Our certificate of incorporation, bylaws and Delaware law contain provisions that could have the effect of rendering more difficult, delaying or preventing an acquisition deemed undesirable by our board of directors or otherwise impact our corporate governance. Our corporate governance documents include provisions:
| ● | authorizing “blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend, and other rights superior to our common stock; | |
| ● | limiting the liability of, and providing indemnification to, our directors and officers; | |
| ● | limiting the ability of our stockholders to call and bring business before special meetings; | |
| ● | requiring advance notice of stockholder proposals for business to be conducted at meetings of our stockholders and for nominations of candidates for election to our board of directors; | |
| ● | controlling the procedures for the conduct and scheduling of board of directors and stockholder meetings; | |
| ● | providing our board of directors with the express power to postpone previously scheduled annual meetings and to cancel previously scheduled special meetings; and
| |
| ● | providing for a quorum comprised of stockholders representing one-third of the voting power of our outstanding shares of common stock to hold valid annual or special meetings of our stockholders. |
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. Our reduced quorum requirement could also lead to corporate governance changes resulting from stockholder required votes in which a minority of one-third of our outstanding stockholders could take actions at annual or special meetings of our stockholders that impact our company as compared to other companies in which a majority of stockholders are required for a quorum.
As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the Delaware General Corporation law, which prevents some stockholders holding more than 15% of our outstanding common stock from engaging in certain business combinations without approval of the holders of substantially all of our outstanding common stock.
Any provision of our certificate of incorporation, bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock. These provisions and the impact of the reduced quorum requirement could also affect the price that some investors are willing to pay for our common stock.
Our certificate of incorporation, as amended, designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees.
Our certificate of incorporation requires that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will, to the fullest extent permitted by law, be the sole and exclusive forum for each of the following:
| ● | any derivative action or proceeding brought on our behalf; | |
| ● | any action asserting a claim for breach of any fiduciary duty owed by any director, officer or other employee of ours to the Company or our stockholders, creditors, or other constituents; | |
| ● | any action asserting a claim against us or any director or officer of ours arising pursuant to, or a claim against us or any of our directors or officers, with respect to the interpretation or application of any provision of, the DGCL, our certificate of incorporation, or bylaws; or | |
| ● | any action asserting a claim governed by the internal affairs doctrine; |
| 83 |
provided that if and only if the Court of Chancery of the State of Delaware dismisses any of the foregoing actions for lack of subject matter jurisdiction, any such action or actions may be brought in another state court sitting in the State of Delaware.
The exclusive forum provision is limited to the extent permitted by law, and it will not apply to claims arising under the Exchange Act or for any other federal securities laws which provide for exclusive federal jurisdiction.
Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, our second amended and restated certificate of incorporation provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring such a claim arising under the Securities Act against us, our directors, officers, or other employees in a venue other than in the federal district courts of the United States of America. In such instance, we would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of our second amended and restated certificate of incorporation.
Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, this provision may limit or discourage a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
We note that there is uncertainty as to whether a court would enforce the provision and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Although we believe this provision benefits us by providing increased consistency in the application of Delaware law in the types of lawsuits to which it applies, the provision may have the effect of discouraging lawsuits against our directors and officers.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
Risk Management and Strategy
We maintain standard procedures to help assess, identify and manage material risk posed by cybersecurity threats and regularly evaluate how we can integrate these procedures into our overall risk management processes. For example, we require that all of our employees who have access to our internal network complete formal cybersecurity training upon hire and on a periodic basis, including training on phishing, malware, and other cybersecurity risks. We also continuously evaluate our information technology systems and our practices that relate to our information technology systems. To date, we have not engaged any assessors, consultants, auditors or other third parties in connection with these efforts but may elect to do so in the future.
To the extent we identify areas in our information systems that need improvement, we seek to timely implement and monitor such improvements. While we believe that we have taken appropriate security measures to protect our data and information technology systems, and have been informed by our third-party vendors that they have as well, there can be no assurance that our efforts will prevent breakdowns or breaches in our systems, or those of our third-party vendors, that could materially adversely affect our business and financial condition. For additional information regarding whether risks from cybersecurity threats are reasonably likely to materially affect us, see Item 1A, “Risk Factors — Security threats to our information technology infrastructure and/or our physical buildings could expose us to liability and damage our reputation and business” in this Report.
| 84 |
Governance
One of the functions of our Audit Committee is to identify principal risks related to our company and ensure implementation of appropriate systems to manage these risks, including risks from cybersecurity threats. Our Audit Committee works with members of our management to identify and manage these risks, including cybersecurity risks. We currently outsource a qualified Information Technology Administrator who reports to our Chief Executive Officer. This consultant has over 25 years of experience with cybersecurity, information technology development and deployment and information technology risk assessment and management, including information security management. Our Information Technology Administrator regularly monitors our information technology systems and monitors the prevention, detection, mitigation and remediation of cybersecurity incidents in consultation with our Chief Executive Officer. To the extent necessary, our Chief Executive Officer reports such risks to our Board of Directors.
ITEM 2. PROPERTIES
Our administrative headquarters is located at 4546 El Camino Real, Suite B1, Los Altos, CA 94022. The office space is approximately 1,480 square feet, and we rent on a month-to-month basis. We believe that our facility is adequate for our current operations and purposes, and that suitable additional or alternative space will be available in the future on commercially reasonable terms, if required.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we may be involved in various legal actions, including claims and proceedings arising in the ordinary course of business. We are currently not a party to any legal proceedings, the adverse outcome of which, in management’s opinion, individually or in the aggregate, would have a material adverse effect on our results of operations or financial position, and, to the best of management’s knowledge, no such litigation is currently pending or threatened.
Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, negative publicity, reputational harm and other factors.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information for Common Stock
Our common stock is publicly traded on the Nasdaq Capital Market under the symbol “RNXT.”
Holders of Record of Common Stock
As of March 25, 2024, there were approximately 120 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. As of such date, there were 16,865,975 shares of our common stock issued and outstanding.
| 85 |
Dividend Policy
We have not declared or paid any cash dividends on our common stock since our inception. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business, and do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our Board of Directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our Board of Directors may deem relevant.
Securities Authorized for Issuance Under Equity Compensation Plans
Information regarding our securities authorized for issuance under equity compensation plans will be included in Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters,” of this Report.
Recent Sales of Unregistered Securities
On January 26, 2024, we have raised additional funding from private placements of shares of common stock and common stock purchase warrants for net offering proceeds of $5.5 million.
Issuer Purchases of Equity Securities
None.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations for our fiscal year ended December 31, 2023 should be read in conjunction with the financial statements and related notes thereto included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this Report. All information presented herein is based on our fiscal year. Unless otherwise stated, references to particular years, quarters, months or periods refer to our fiscal years ended December 31 and the associated quarters, months and periods of those fiscal years.
Some of the information contained in this discussion and analysis or set forth elsewhere in this Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involves risks and uncertainties. See “Cautionary Note Regarding Forward-Looking Statements” and “Risk Factors” for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements.
Unless the context otherwise requires, all references in this section to the “Company,” “we,” “us,” or “our” refer to RenovoRx, Inc.
Overview
We are a clinical-stage biopharmaceutical company developing proprietary targeted combination therapies for high unmet medical needs with a goal to improve therapeutic outcomes for cancer patients undergoing treatment. Our proprietary Trans-Arterial Micro-Perfusion (TAMP) therapy platform is designed to ensure precise therapeutic delivery to directly target the tumor while potentially minimizing a therapy’s toxicities versus systemic (intravenous (known as “IV”) therapy).
Our novel and patented approach to targeted treatment offers the potential for increased safety, tolerance, and improved efficacy. Our Phase III lead product candidate, RenovoGem, an oncology drug-device combination product, is being investigated under a U.S. Investigational New Drug Application (“IND”) that is regulated by the U.S. Food and Drug Application (“FDA”) 21 CFR 312 pathway. RenovoGem is currently being evaluated for the treatment of locally advanced pancreatic cancer (“LAPC”) by the Center for Drug Evaluation and Research (the drug division of FDA). We also plan to evaluate RenovoGem as a potential therapy in bile duct cancer with other potential pipeline indication opportunities to follow including non-small cell lung cancer, uterine tumors, glioblastoma, and sarcoma.
RenovoGem received FDA Orphan Drug Designation for pancreatic cancer and bile duct cancer, which provides 7 years of market exclusivity upon approval by the FDA of our New Drug Application (“NDA”) for RenovoGem Should such approval be obtained.
We have completed our RR1 Phase I/II and RR2 observational registry studies of RenovoGem, with 20 and 25 patients respectively, in LAPC. These studies demonstrated a median Overall Survival (known as “OS”) of 27.9 months in LAPC patients pre-treated with radiation followed by treatment with RenovoGem. Based on previous large randomized clinical trials, the expected survival of LAPC patients is 12 to 15 months in patients receiving only IV systemic chemotherapy or IV chemotherapy plus radiation (which are both considered standard of care). Unlike the randomized trials that established these standard of care results, our RR1 and RR2 clinical trials did not prospectively control the standard of care therapy received prior to administration of RenovoGem. Based on an FDA safety review of our Phase I/II study, FDA allowed us to proceed to evaluate RenovoGem within our Phase III registrational clinical trial.
| 86 |
Our Phase III registrational trial of RenovoGem for the treatment of LAPC is called TIGeR-PaC. This clinical trial is an ongoing randomized multi-center study using TAMP to evaluate RenovoGem. The study is evaluating trans-arterial delivery, a form of intra-arterial (“IA”) administration, of an FDA-approved chemotherapy, gemcitabine, to treat LAPC following stereotactic body radiation therapy (“SBRT”). The study is comparing treatment of an FDA-approved cancer drug, gemcitabine, with TAMP versus systemic IV administration of gemcitabine and nab-paclitaxel.
Funding permitting, we also plan to evaluate RenovoGem as a potential therapy in bile duct cancer with other potential pipeline indication opportunities to follow including non-small cell lung cancer, uterine tumors, glioblastoma, and sarcoma.
Since our inception, we have devoted substantially all of our efforts to developing our cancer therapy platform and product candidates, raising capital and organizing and staffing our Company. As of December 31, 2023, we have received over $41.9 million in gross proceeds and have financed our operations primarily through issuance of convertible preferred stock, convertible notes, securities in our IPO, common stock purchase warrants, a registered direct offering in April 2023 and a loan pursuant to the Paycheck Protection Program under the Coronavirus Aid, Relief and Economic Security Act (the CARES Act). After deducting underwriting discounts, commissions, placement fees, legal fees, and other professional expenses of $3.6 million, our net offering proceeds were $38.3 million. Subsequent to December 31, 2023, we have raised additional funding from private placements of shares of common stock and common stock purchase warrants for net offering proceeds of $5.5 million.
We have incurred significant operating losses and generated negative cash flows from operations since our inception. As of December 31, 2023, we had cash and cash equivalents of $1.2 million. We had net losses of $10.2 million and $9.9 million for the years ended December 31, 2023, and 2022, respectively. As of December 31, 2023, we had an accumulated deficit of $41.4 million. We expect to continue to incur significant expenses, increasing operating losses and negative cash flows for the foreseeable future. We do not expect to generate revenues from product sales unless and until we successfully complete development and obtain regulatory approval for one or more product candidates. Given economic and market conditions and timing of regulatory approval, we expect that our expenses will increase in connection with our ongoing research and development activities, particularly if and when we decide to:
| ● | Advance clinical development of RenovoGem and our platform technology by continuing to enroll patients in our ongoing Phase III TIGeR-PaC clinical trial, expanding the number of clinical trials including our potential clinical trial in eCCA, and advancing RenovoGem through preclinical and clinical pipeline indication opportunities; | |
| ● | Hire additional research, development, engineering, and general and administrative personnel; | |
| ● | Pursue collaborations, licensing arrangements or other strategic or commercial activities relating to our technology; | |
| ● | Maintain, expand, enforce, defend, and protect our intellectual property portfolio; and | |
| ● | Expand our operational, financial and management systems and increase personnel, including personnel to support our clinical development, manufacturing and commercialization efforts and our operations as a public company. |
| 87 |
In addition to the variables described above, if and when any of our product candidates successfully complete development, we will incur substantial additional costs associated with establishing a sales, marketing, medical affairs and distribution infrastructure to commercialize products for which we may obtain marketing approval, regulatory filings, marketing approval, and post-marketing requirements, in addition to other commercial costs. We cannot reasonably estimate these costs at this time.
Due to our recurring operating losses and the expectation that we will continue to incur net losses in the future, we will be required to raise additional capital to complete the development and commercialization of our product candidates. We have historically financed our operations primarily through private sales of our equity, debt financing and the sale of common stock and warrants in our IPO. To raise additional capital, we may seek to sell additional equity and/or debt securities, obtain a credit facility or other loan or enter into collaborations, licenses or other similar arrangements, which we may not be able to do on favorable terms, or at all.
On January 26, 2024, we completed a private placement to 92 accredited investors with gross proceeds of $6.1 million. The private placement included issuing 6,133,414 shares of our common stock including 6,133,414 million common stock warrants, expiring five years from the date, January 26, 2024, to purchase additional common shares. In connection with the private placement, we entered into a placement agent agreement, as additional compensation to the placement agent, and issued 511,940 common stock warrants expiring five years from the settlement date.
Our ability to obtain additional financing will be subject to a number of factors, including market conditions, fluctuations in interest rates, our operating performance and investor sentiment. If we are unable to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue the development and/or commercialization of our product candidates, restrict or cease our operations or obtain funds by entering into agreements on unfavorable terms. Failure to obtain additional capital on acceptable terms, or at all, would result in a material and adverse impact on our operations. As a result, there is substantial doubt about our ability to operate as a going concern.
Our financial statements as of December 31, 2023 have been prepared on a going concern basis and do not include any adjustments that may result from the outcome of this uncertainty. Based on our operating plans, we expect that our current cash and cash equivalents as of the date of this Report will be sufficient to fund our operating, investing and financing cash flow needs through the fourth quarter of 2024, assuming our programs advance as currently contemplated.
As a result, we will require significant additional funding to support our continuing operations. Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through private or public equity financings, debt financings and collaborations, licenses or other similar arrangements. We currently have no credit facility or committed sources of capital. To the extent that we raise additional capital through the future sale of equity or debt, the ownership interests of our stockholders will be diluted and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing common stockholders. If we raise additional funds through the issuance of debt securities, these securities could contain covenants that would restrict our operations. We may require additional capital beyond our currently anticipated amounts and additional capital may not be available on reasonable terms, or at all. If we raise additional funds through collaboration arrangements or other strategic transactions in the future, we may have to relinquish valuable rights to our technologies or future revenue streams or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through private or public equity financings or debt financings when needed, we may be required to delay, limit, reduce or terminate development or future commercialization efforts, and we may be unable to continue as a going concern. If we are unable to continue as a going concern, we might have to liquidate our assets and the value we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements, and our shareholders may lose their entire investment in our common stock.
| 88 |
Components of Our Results of Operations
Revenue
We have not generated any revenue from product sales and do not expect to generate any revenue from the sale of products for several years, if at all. If our development efforts for our current or future product candidates are successful and result in marketing approval or collaboration or license agreements with third parties, we may generate revenue in the future from a combination of product sales or payments from collaboration or license agreements.
Operating Expenses
Research and Development
Research and development expenses consist of costs related to the research and development of our platform technology. Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors and consultants. We outsource a substantial portion of our clinical trial activities, utilizing the service of third-party clinical trial sites and contract research organizations to assist us with the execution of our clinical trials. In addition, we have FDA 510(k) clearance for the RenovoCath delivery device, which comprises part of the RenovoGem product. Accordingly, we are able to charge our clinical trial sites for the RenovoCath delivery device. To date, payments from clinical trial sites in consideration for RenovoCath delivery devices have been adequate to cover our direct manufacturing costs. Any payments we receive from clinical trial sites as consideration for use of RenovoCath delivery devices offset our research and development expenses. We expect our research and development expenses to increase for the foreseeable future as we continue the development of our product candidates and enroll subjects in our ongoing Phase III clinical trial, initiate new clinical trials and pursue regulatory approval of our product candidates. It is difficult to predict with any certainty the duration and costs of completing our current or future clinical trials of our product candidates or if, when or to what extent we will achieve regulatory approval and generate revenue from the commercialization and sale of our product candidates. The duration, costs and timing of clinical trials and other development of our product candidates will depend on a variety of factors, including uncertainties in clinical trial enrollment, timing and extent of future clinical trials, development of new product candidates and significant and changing government regulation. We may never succeed in achieving regulatory approval for any of our product candidates.
Our research and development expenses include:
| ● | expenses incurred under agreements with clinical trial sites, contract research organizations, and consultants that are involved in conducting our clinical trials; | |
| ● | costs of acquiring and developing clinical trial materials; | |
| ● | personnel costs, including salaries, benefits, bonuses, and stock-based compensation for employees engaged in preclinical and clinical research and development; | |
| ● | costs related to compliance with regulatory requirements; | |
| ● | third-party vendor costs related to manufacturing materials and testing; | |
| ● | costs related to preclinical studies and pilot testing; | |
| ● | travel expenses; and | |
| ● | allocated general and administrative expenses which includes facilities and other indirect administrative expenses to support research and development activities. |
Research and development costs are expensed as incurred. Costs for certain development activities, such as clinical trials and preclinical studies, are recognized based on evaluation of progress to completion of specific tasks using data such as subject enrollment, clinical site activations or information provided to us by third party vendors.
| 89 |
General and Administrative
General and administrative expenses consist of salaries, benefits, and stock-based compensation for personnel in executive, finance and administrative functions, professional services and associated costs related to accounting, tax, audit, legal, intellectual property and other matters, consulting costs, conferences, travel and allocated expenses for rent, insurance and other general overhead costs. We expect to continue to incur additional expenses as a result of operating as a public company, including costs to comply with the rules and regulations of the Securities and Exchange Commission, or SEC, and Nasdaq listing standards and increased expenses in the areas of insurance, professional services and investor relations. As a result, we expect our general and administrative expenses to increase in the foreseeable future. General and administrative expenses are expensed as incurred.
Other Income (Expenses), Net
Interest Income (Expense) Net
Interest expense consists of expense for the amortization of Directors and Officers liability insurance premiums.
Interest income is earned from cash deposited in our short-term marketable securities and money market account.
Change in Fair Value of Warrant Liability
Change in fair value of warrant liability represents the gain or loss reported from the change in the fair value of the common warrant liability for warrants issued under the registered direct offering. On April 3, 2023, we completed a registered direct offering financing issuing common shares and common warrants. The fair value of the common warrant per share was $2.77 and $1.69 on April 3, 2023 and December 31, 2023, respectively. The decrease in the fair value was primarily due to the decrease in our stock price.
Transaction Costs Allocated to Common Warrant Liability
Direct offering costs incurred on our registered direct financing consist principally of agency placement fees, legal and other professional expenses.
Income Tax Expense
We account for income taxes using the asset and liability method. Under this method, deferred income tax assets and liabilities are recorded based on the estimated future tax effects of differences between the financial statement and income tax basis of existing assets and liabilities. Deferred income tax assets and liabilities are recorded net and classified as noncurrent on the balance sheets. A valuation allowance is provided against our deferred income tax assets when their realization is more likely than not.
We are subject to income taxes in the federal and state jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. In accordance with the authoritative guidance on accounting for uncertainty in income taxes, we recognize tax liabilities for uncertain tax positions when it is more likely than not that a tax position will not be sustained upon examination and settlement with various taxing authorities. Liabilities for uncertain tax positions are measured based upon the largest amount of benefit that is more-likely-than-not (greater than 50%) of being realized upon settlement. Our policy is to recognize interest and/or penalties related to income tax matters in income tax expense.
On March 27, 2020, the CARES Act was enacted. The CARES Act includes several significant provisions for corporations, including the usage of net operating losses, interest deductions and payroll benefits. Corporate taxpayers may carryback net operating losses, or NOLs, originating during 2018 through 2020 for up to five years.
| 90 |
Results of Operations
Comparison of the Years Ended December 31, 2023 and 2022
The following table summarizes the significant components of our results of operations for the periods presented (in thousands, except percentages):
| Years Ended December 31, | Increase / (Decrease) | |||||||||||||||
| 2023 | 2022 | $ | % | |||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | $ | 5,667 | $ | 4,301 | $ | 1,366 | 32 | % | ||||||||
| General and administrative | 5,729 | 5,649 | 80 | 1 | % | |||||||||||
| Total operating expenses | 11,396 | 9,950 | 1,446 | 15 | % | |||||||||||
| Loss from operations | (11,396 | ) | (9,950 | ) | (1,446 | ) | 15 | % | ||||||||
| Other income (expense), net | ||||||||||||||||
| Interest income (expense), net | 108 | 57 | 51 | 89 | % | |||||||||||
| Other income (expense), net | - | 4 | (4 | ) | n/a | |||||||||||
| Change in fair value of common warrant liability | 1,709 | - | 1,709 | n/a | ||||||||||||
| Transaction costs allocated to common warrant liability | (653 | ) | - | (653 | ) | n/a | ||||||||||
| Total other income (expense), net | 1,164 | 61 | 1,103 | 1808 | % | |||||||||||
| Net loss | $ | (10,232 | ) | $ | (9,889 | ) | $ | (343 | ) | (3 | )% | |||||
Research and Development
The following table summarizes our research and development expenses (in thousands):
| Years Ended December 31, | Increase / (Decrease) | |||||||||||
| 2023 | 2022 | $ | ||||||||||
| Preclinical research and development | $ | 1,498 | $ | 1,861 | $ | (363 | ) | |||||
| Clinical development | 2,842 | 1,548 | 1,294 | |||||||||
| Personnel | 1,072 | 690 | 382 | |||||||||
| Regulatory | 351 | 343 | 8 | |||||||||
| Clinical site payments for RenovoCath devices | (96 | ) | (141 | ) | 45 | |||||||
| Total research and development | $ | 5,667 | $ | 4,301 | $ | 1,366 | ||||||
Research and development expenses were $5.7 million for the year ended December 31, 2023 compared to $4.3 million for the year ended December 31, 2022, an increase of $1.4 million. The period-over-period increase in research and development expenses is primarily driven by $1.3 million increase in clinical development expense, which includes an increase of $0.7 million for additional Phase III clinical sites and new patient enrollments and an increase of $0.5 million for clinical consultants, an increase of $0.4 million in personal expense for employees and benefits, offset by a decrease of $0.4 million in preclinical research and development expense, which includes a decrease of $0.4 million in costs associated with a secondary manufacturer of RenovoCath delivery devices. Regulatory expense and cash payments made for use of RenovoCath delivery devices used in Phase III clinical trial were relatively flat for the year ended December 31, 2023 compared to the previous year. Payments received from clinical trial sites for the devices have been adequate to cover our direct costs of manufacturing the RenovoCath delivery devices and to offset research and development expenses. We expect the costs of research and development to remain at the current levels next year as we continue to target our efforts on our Phase III TIGeR-PaC clinical trial study, including the preparation of second interim analysis which will be triggered by the 52nd event (death of patient), estimated to occur in late 2024.
| 91 |
General and Administrative Expenses
The following table summarizes our general and administrative expenses (in thousands):
| Years Ended December 31, | Increase / (Decrease) | |||||||||||
| 2023 | 2022 | $ | ||||||||||
| Professional services and other | $ | 2,726 | $ | 3,166 | $ | (440 | ) | |||||
| Personnel | 2,468 | 1,916 | 552 | |||||||||
| Legal fees | 535 | 567 | (32 | ) | ||||||||
| Total general and administrative | $ | 5,729 | $ | 5,649 | $ | 80 | ||||||
General and administrative expenses were $5.7 million for the year ended December 31, 2023 compared to $5.6 million for the year ended December 31, 202, an increase of $0.1 million. The period-over-period increase in general and administrative expenses was primarily attributable to an increase of $0.6 million in personnel expense primarily due to salaries and benefits recognized for a full year vs. a partial year in fiscal year 2022, offset by a decrease of $0.4 million in professional services and other, including incurring $0.3 million less in consulting fees, $0.3 million less in directors and officers insurance, $0.3 million less in office support, $0.2 million increase in the allocation of general and administrative expenses to research and development, offset by a $0.8 million increase in investor and public relation services. Legal fees expense was relatively flat for the year ended December 31, 2023 compared to the previous year. We expect general and administrative expenses to be lower in the next fiscal year as we reduce spending in all three subgroups above.
Interest (Expense) Income, Net (in thousands)
| Years Ended December 31, | Increase / (Decrease) | |||||||||||
| 2023 | 2022 | $ | ||||||||||
| Interest income (expense), net | $ | 108 | $ | 57 | $ | 51 | ||||||
Interest income was $0.1 million for the years ended December 31, 2023, and December 31, 2022. Interest income represents income earned on excess operating cash invested in short-term U.S. Treasury bills.
Change in Fair Value of Common Warrant Liability (in thousands)
| Years Ended December 31, | Increase / (Decrease) | |||||||||||
| 2023 | 2022 | $ | ||||||||||
| Change in fair value of common warrant liability | $ | 1,709 | $ | - | $ | 1,709 | ||||||
The change in fair value of common warrant liability was $1.7 million for the year ended December 31, 2023. The increase was primarily due to a $1.7 million change in the fair value of the common warrant due to a decrease in our common stock price during the period.
Transaction Costs Allocated to Warrant Liability (in thousands)
| Years Ended December 31, | Increase / (Decrease) | |||||||||||
| 2023 | 2022 | $ | ||||||||||
| Transaction costs allocated to warrant liability | $ | (653 | ) | $ | - | $ | (653 | ) | ||||
Transaction costs allocated to the common warrants from the Registered Direct Offering (“RDO”) were $0.7 million.
| 92 |
Liquidity and Capital Resources
For the years ended December 31, 2023 and December 31, 2022, our net losses were $10.2 million and $9.9 million, respectively. As of December 31, 2023, we had an accumulated deficit of $41.4 million. Our primary requirements for liquidity have been to fund our clinical trial activity and general corporate and working capital needs.
On April 3, 2023, we completed the RDO under our shelf registration statement on Form S-3 for the purchase and sale of 1,557,632 shares of Common Stock (or Pre-funded Common Warrants) at a purchase price of $3.21 per share of Common Stock (or Pre-Funded Common Warrants) to a certain institutional investor. Additionally, in a concurrent private placement, we issued to the investor Common Warrants to purchase up to 1,947,040 shares of its Common Stock. The aggregate gross proceeds from the March 2023 Offering were $5.0 million, and the net offering proceeds were $4.3 million after deducting placement agent fees and placement agent’s expenses of $0.4 million and other professional expenses of $0.3 million.
As of December 31, 2023, we have received over $41.9 million in gross proceeds through various financial arrangements to include the issuance of preferred stock, convertible debt, securities in our IPO, common warrants, RDO and other various loans. After deducting underwriting discounts, commissions, placement fees, legal fees, and other professional expenses of $3.6 million, our net offering proceeds were $38.3 million.
Based on our operating plans, we do not expect that our current cash and cash equivalents as of December 31, 2023, will be sufficient to fund our operating, investing and financing cash flow needs for at least the next twelve months, assuming our programs advance as currently contemplated. Based upon this review and our current financial condition, the Company has concluded that substantial doubt exists as to our ability to continue as a going concern. We have (including our private placement in January 2024 as described below) and believe we will continue to be able to raise additional capital through debt financing, private or public equity financings, license agreements, collaborative agreements or other arrangements with other companies, or other sources of financing. However, there can be no assurances that such financing will be available or will be at terms acceptable to us, or at all. If we are unable to raise capital when needed or on attractive terms, we would be forced to delay, reduce or eliminate our clinical trials or other operations. If any of these events occur, our ability to achieve our operational goals would be adversely affected. Our future capital requirements and the adequacy of available funds will depend on many factors, including those described in the section titled “Risk Factors.” Depending on the severity and direct impact of these factors on us, we may be unable to secure additional financing to meet our operating requirements on commercially acceptable terms favorable to us, or at all.
Sources of Liquidity
Since our inception, we have not generated any revenue from product sales and we have incurred significant operating losses and negative cash flows from operations. We anticipate that we will continue to incur net losses for the foreseeable future. We do not have any products that have achieved regulatory marketing approval and we do not expect to generate revenue from sales of any product candidates for several years, if ever.
On January 26, 2024, we completed a private placement to 92 accredited investors with gross proceeds of $6.1 million. The private placement included issuing 6,133,414 shares of our common stock including 6,133,414 million common stock warrants, expiring five years from the date, January 26, 2024, to purchase additional common shares. In connection with the private placement, we entered into a placement agent agreement, as additional compensation to the placement agent, and issued 511,940 common stock warrants expiring five years from the settlement date. See “Note 12. Subsequent Events” in Notes to Financial Statements.
We have financed our operations primarily through the issuance and sale of convertible preferred stock and convertible debt. Through the date of this report, we have raised an aggregate of $48.0 million from private placements of our convertible preferred stock, convertible debt securities, the issuance of securities in our IPO, various loans and the exercise of warrants and common stock options.
| 93 |
Cash Flows
Our primary uses of cash are to fund our operations including research and development and general and administrative expenses. We will continue to incur operating losses in the future and expect that our research and development and general and administrative expenses will continue to increase as we continue our research and development efforts with respect to clinical development of our product candidates and further develop our platform. We have used a substantial portion of the net proceeds of the IPO, in combination with our existing cash and cash equivalents, for these purposes and for the increased expenses associated with being a public company. Cash used to fund operating expenses is impacted by the timing of when we pay expenses, as reflected in the change in our outstanding accounts payable and accrued expenses.
The following table summarizes our cash flows for the periods indicated (in thousands):
| 2023 | 2022 | |||||||
| Net cash (used in) provided by: | ||||||||
| Operating activities | $ | (10,258 | ) | $ | (8,811 | ) | ||
| Investing activities | 2,032 | (2,032 | ) | |||||
| Financing activities | 5,008 | 42 | ||||||
| Decrease in cash and cash equivalents | $ | (3,218 | ) | $ | (10,801 | ) | ||
Cash Used in Operating Activities
Net cash used in operating activities was $10.3 million for the year ended December 301, 2023, which was primarily attributable to our net loss of $10.2 million and noncash charges of $0.6 million, adjusted for net changes in operating assets and liabilities of $0.6 million. Noncash charges included primarily of stock-based compensation expense, and loss on the fair value of common warrants. Net cash used in operating activities for the year ended December 31, 2022 reflected a net loss of $9.9 million adjusted for a net change in our operating assets and liabilities of $0.4 million, offset by net non-cash charges of $0.7 million consisting primarily of stock-based compensation expense.
Cash Provided By/Used in Investing Activities
Net cash provided by investing activities for the year ended December 31, 2023 was $2.0 million consisted of proceeds of U.S. Treasury bills, which are classified as available-for-sale securities. Net cash used in investing activities for the year ended December 31, 2022 was $2.0 million consisted of purchases and proceeds of U.S. Treasury bills, which are classified as available-for-sale securities.
Cash Provided by Financing Activities
Net cash provided by financing in the year ended December 31, 2023 was $5.0 million, consisting primarily of proceeds of 5.0 million from the issuance of common stock in our RDO. Net cash provided by financing in the year ended December 31, 2022, was $42,000 consisted of exercise of stock options.
Contractual Obligations and Other Commitments
There have been no significant changes in our contractual obligations or other commitments as of December 31, 2023. As of the date of this Report, we have no contractual obligations or other commitments.
Critical Accounting Policies and Significant Judgments and Estimates
The accompanying management’s discussion and analysis of our financial condition and results of operations are based upon our financial statements and the related disclosures, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts in our financial statements and accompanying notes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. To the extent that there are material differences between these estimates and actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected. While our significant accounting policies are described in the notes to our financial statements included elsewhere in this report, we believe that the following critical accounting policies are most important to understanding and evaluating our reported financial results because they require us to make estimates, assumptions and judgments about matters that are inherently uncertain.
| 94 |
A critical accounting policy is defined as one that is both material to the presentation of our financial statements and requires management to make difficult, subjective, or complex judgments that could have a material effect on our financial condition and results of operations. Specifically, critical accounting estimates have the following attributes: (i) we are required to make assumptions about matters that are highly uncertain at the time of the estimate; and (ii) different estimates we could reasonably have used, or changes in the estimate that are reasonably likely to occur, would have a material effect on our financial condition or results of operations.
Clinical Trial Expenses
We make payments in connection with our Phase III clinical trial under contracts with clinical trial sites and contract research organizations that support conducting and managing clinical trials. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Generally, these agreements set forth the scope of work to be performed at a fixed fee, unit price or on a time and materials basis. A portion of the obligation to make payments under these contracts depends on factors such as the successful enrollment or treatment of patients or the completion of other clinical trial milestones.
Expenses related to clinical trials are accrued based on estimates and/or representations from service providers regarding work performed, including actual level of patient enrollment, completion of patient studies and progress of the clinical trials. Other incidental costs related to patient enrollment or treatment are accrued when reasonably estimable. If the amounts we are obligated to pay under clinical trial agreements are modified (for instance, as a result of changes in the clinical trial protocol or scope of work to be performed), the accruals are adjusted accordingly. Revisions to contractual payment obligations are charged to expense in the period in which the facts that give rise to the revision become reasonably certain.
Stock-Based Compensation
We estimate the fair value of stock options using the Black-Scholes option pricing model, which incorporates various assumptions including those related to the fair value of our common stock, volatility, expected term, and risk-free interest rate. Compensation related to service-based awards is recognized starting on the grant date on a straight-line basis over the vesting period, which is generally four years, see “Note 7. Equity Incentive Plan – Stock-Based Compensation and Common Stock Warrants” in Notes to Financial Statements.
Determining the grant date fair value of options using the Black-Scholes option pricing model requires management to make assumptions and judgments. If any of the assumptions used in the Black-Scholes model change significantly, stock-based compensation for future awards may differ materially compared with the awards granted previously. The assumptions and estimates are as follows:
Fair Value of Common Stock—Given the absence of a public trading market, pre-IPO, our Board considered numerous objective and subjective factors to determine the fair value of our common stock at each grant date. These factors included but were not limited to: (i) contemporaneous third-party valuations of common stock; (ii) the prices for preferred stock sold to outside investors; (iii) the rights and preferences of preferred stock relative to common stock; (iv) the lack of marketability of our common stock; (v) developments in the business; and (vi) the likelihood of achieving a liquidity event, such as an IPO or sale of the business, given prevailing market conditions. The methodology to determine the fair value of our common stock included estimating the fair value of the enterprise using the “backsolve” method, which is a market approach that assigns an implied enterprise value by accounting for all share class rights and preferences based on the latest round of financing. The total equity value implied was then applied in the context of an option pricing model to determine the value of each class of our shares.
For grants issued post-IPO, we rely on the closing price of our common stock as reported on the date of grant to determine the fair value of our common stock, as shares of our common stock are traded in the public market.
Expected Term—The expected term represents the period that the stock-based awards are expected to be outstanding. We determine the expected term using the simplified method for pre-IPO and post-IPO awards. The simplified method deems the term to be the average of the time-to-vesting and the contractual life of the options.
| 95 |
Expected Volatility—Given the absence of a public trading market, pre-IPO and post IPO, the expected volatility was estimated by taking the average historic price volatility for industry peers, consisting of several public companies in our industry that are either similar in size, stage, or financial leverage, over a period equivalent to the expected term of the awards.
Risk-Free Interest Rate—The risk-free interest rate is calculated using the average of the published interest rates of U.S. Treasury zero-coupon issues with maturities that are commensurate with the expected term.
Dividend Rate—The dividend yield assumption is zero as we have no plans to make dividend payments.
Convertible Instruments and Embedded Derivatives
We evaluate all of our agreements to determine whether such instruments have derivatives or contain features that qualify as embedded derivatives. We account for certain redemption features that are associated with the terms of convertible notes as liabilities at fair value and adjusts the instruments to their fair value at the end of each reporting period. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in other income (expenses), net in the statements of operations. Derivative instrument liabilities are classified in the balance sheets as current or non-current based on whether or not net-cash settlement of the derivative instrument could be required within 12 months of the balance sheet date.
Pre-Funded Common Warrants and Common Warrants
We evaluate Pre-Funded Common Warrants and Common Warrants issued in connection direct financing to determine whether such warrants qualify for equity classification, or meet the definition of a derivative instrument, classified as a liability on the Balance Sheets and measured at fair value at inception and at each reporting date with changes in fair value recognized in the Statements of Operations and Comprehensive Loss in the period of change.
Direct Offering Costs
Direct offering costs consist principally of commissions, placement fees and other expenses, including other professional expenses incurred. We evaluate the terms under the financing agreement to determine the classification of direct costs in the accompanying Balance Sheets or accompanying Statements of Operations and Comprehensive Loss or some combinations of both.
Emerging Growth Company and Smaller Reporting Company Status
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended, or the JOBS Act. Under the JOBS Act, companies have extended transition periods available for complying with new or revised accounting standards. We have elected this exemption to delay adopting new or revised accounting standards. We will remain an emerging growth company until the earlier of (1) December 31, 2026, (2) the last day of the fiscal year in which we have total annual gross revenues of at least $1.07 billion, (3) the date on which we are deemed to be a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, or (4) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period. An emerging growth company may take advantage of specified reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. As an emerging growth company,
| ● | we may present only two years of audited financial statements, plus unaudited condensed financial statements for any interim period, and related Management’s Discussion and Analysis of Financial Condition and Results of Operations; | |
| ● | we may avail ourselves of the exemption from the requirement to obtain an attestation and report from our auditors on the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act; |
| 96 |
| ● | we may provide reduced disclosure about our executive compensation arrangements; and | |
| ● | we do not require stockholder non-binding advisory votes on executive compensation or golden parachute arrangements. |
We have elected to take advantage of certain of the reduced disclosure obligations in this Annual Report on Form 10-K and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
We are also a “smaller reporting company,” meaning that the market value of our stock held by non-affiliates plus the proposed aggregate amount of gross proceeds to us as a result of this offering is less than $700.0 million and our annual revenue is less than $100.0 million during the most recently completed fiscal year. We may continue to be a smaller reporting company if either (1) the market value of our stock held by nonaffiliates is less than $250.0 million or (2) our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700.0 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, like emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Recently Issued and Adopted Accounting Pronouncements
See “Note 2. Summary of Significant Accounting Policies” in Notes to Financial Statements, to our audited financial statements included elsewhere in this Report for more information.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The disclosures in this section are not required because we qualify as a smaller reporting company under federal securities laws.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Our financial statements and the report of our independent registered public accounting firm, as listed under Part IV, Item 15. “Exhibits and Financial Statement Schedules,” are included as a separate section of this Report beginning on page F-1 and are incorporated herein by reference.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None
ITEM 9A. CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and Principal Accounting Officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the year ended December 31, 2022. Based on this evaluation, our Chief Executive Officer and Principal Accounting Officer have concluded that, during the period covered by this Report, our disclosure controls and procedures were not effective due to our previously identified material weaknesses in internal control over financial reporting. As a result, we have performed additional analysis as deemed necessary to ensure that our financial statements were prepared in accordance with U.S. GAAP. Accordingly, notwithstanding the identified material weaknesses, management, including our Chief Executive Officer and Principal Accounting Officer, believes the financial statements included in this Report are fairly presented, in all material respects, in accordance with U.S. GAAP.
| 97 |
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated, communicated and discussed with our management, including our Chief Executive Officer and Principal Officer or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure. Management recognizes that controls and procedures, no matter how well designed and operated, can only provide reasonable, not absolute, assurance the desired control objectives will be met. In reaching a reasonable level of assurance, management has weighed the cost of contemplated controls against their intended benefits. The design of any system of controls is based on management’s assumptions about the likelihood of future events. We cannot assure you that our controls will achieve their stated goals under all possible conditions. Changes in future conditions may render our controls inadequate or may cause our degree of compliance with them to deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
(b) Management’s Report on Internal Control over Financial Reporting
For the fiscal year ended December 31, 2023, our management identified a material weakness in our internal control over financial reporting related to our control environment. A material weakness is a deficiency, or combination of significant deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected and corrected on a timely basis.
Specifically, we have determined that we have not maintained adequate formal accounting policies, processes and controls related to complex transactions as a result of a lack of finance and accounting staff with the appropriate GAAP technical expertise needed to identify, evaluate and account for complex and non-routine transactions. We also determined that we have not maintained sufficient staffing or written policies and procedures for accounting and financial reporting, which contributed to the lack of a formalized process or controls for management’s timely review and approval of financial information. More specifically, we have determined that our financial statement close process includes significant control gaps mainly driven by the small size of our accounting and finance staff and, as a result, a significant lack of appropriate segregation of duties. This includes the ability of users to create and post journal entries without adequate compensating review controls as well as review of system rights on the journal entry and financial close process. In addition, we did not have proper information technology general controls (ITGCs) related to user access, including the performance of user access reviews, access to edit data in applications was not properly restricted, and formal approval of application access was not documented and retained.
We are in the process of implementing a number of measures to address the material weaknesses that has been identified including: (i) engaging additional accounting and financial reporting personnel with U.S. GAAP, and SEC reporting experience, (ii) developing, communicating and implementing an accounting policy manual for our accounting and financial reporting personnel for recurring transactions and period-end closing processes, and (iii) establishing effective monitoring and oversight controls for non-recurring and complex transactions to ensure the accuracy and completeness of our financial statements and related disclosures.
These additional resources and procedures are designed to enable us to broaden the scope and quality of our internal review of underlying information related to financial reporting and to formalize and enhance our internal control procedures. With the oversight of senior management and our Audit Committee, we have begun taking steps and plan to take additional measures to remediate the underlying causes of the material weaknesses.
We intend to complete the implementation of our remediation plan when we have sufficient cash to remediate our material weakness. Although we believe that our remediation plan will improve our internal control over financial reporting, additional time may be required to fully implement it and to make conclusions regarding the effectiveness of our internal control over financial reporting. Our management will closely monitor and modify, as appropriate, the remediation plan to eliminate the identified material weakness.
This Report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the Company’s registered public accounting firm due to a transition period established by SEC rules for newly public companies. For as long as we remain an emerging growth company under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting. When we lose our status as an emerging growth company and reach an accelerated filer threshold, our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting.
| 98 |
(c) Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the last fiscal quarter ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Officers
The following table and text set forth the names and ages of our directors and executive officers, as well as certain other officers of our company, as of the date of this Report. Our Board of Directors is comprised of only one class of directors. Also provided herein are brief descriptions of the business experience of each director and executive officer during the past five years (based on information supplied by them) and an indication of directorships held by each director in other public companies subject to the reporting requirements under the federal securities laws.
| Name | Age | Position(s) and Office(s) Held with the Company | ||
| Shaun R. Bagai | 47 | Chief Executive Officer and Director | ||
| Ramtin Agah, M.D. | 58 | Chief Medical Officer and Director | ||
| Kirsten Angela Macfarlane | 59 | Director | ||
| Laurence J. Marton, M.D. | 80 | Director | ||
| Una S. Ryan, O.B.E., Ph.D., D.Sc. | 82 | Director | ||
| Robert Spiegel, M.D. FACP | 74 | Director | ||
| Ronald B. Kocak | 66 | Vice President, Controller and Principal Accounting Officer | ||
| Leesa Gentry | 54 | Chief Clinical Officer |
Shaun R. Bagai. Mr. Bagai has served as our Chief Executive Officer and director since June 2014. Prior to joining us, Mr. Bagai led Global Market Development for HeartFlow, Inc. from 2011 to 2014, which included directing Japanese market research, regulatory/payer collaboration, and Key Opinion Leader development to create value resulting in a company investment to form HeartFlow-Japan. During his tenure at HeartFlow, he successfully orchestrated its largest clinical trial to date and contracted HeartFlow’s first global customers. In addition, Mr. Bagai has launched innovative technologies into regional and global marketplaces in both large corporations and growth-phase novel technology companies. He was instrumental in developing the European market for renal denervation for the treatment of hypertension leading to the acquisition of the first renal denervation company — Ardian, Inc. by Medtronic in 2011. Mr. Bagai is a graduate from the University of California, Santa Barbara with a BSc. in Biology/Pre-Med.
Mr. Bagai brings over eight years of experience leading RenovoRx. During his tenure, we received several FDA clearances and CE Mark for the device component of the drug/device combination, were issued several U.S. and European patents, began to scale production of our catheter delivery system, conducted and reported on our Phase 1/2 and observational registry clinical studies, launched our ongoing Phase 3 study, and completed multiple financings, including our IPO. Mr. Bagai not only has a deep understanding of, and experience with our therapy platform, but also has an extensive background in operations, clinical development and medical device market development and commercial launch, making him well-positioned to lead our management team and provide essential insight to the Board.
| 99 |
Ramtin Agah, M.D. Dr. Agah has served as our Chief Medical Officer and Co-Founder since December 2009, and as Chairman of the Board since May 2018. Dr. Agah is currently an Interventional Cardiologist at El Camino Hospital, Mountain View, a role he began in September 2005. He also has acted as a physician consultant for Abbott Vascular since July 2012. Previously, Dr. Agah was an Assistant Professor of Internal Medicine with the Division of Cardiology, University of Utah. Dr. Agah completed a fellowship in Interventional Cardiology with Cleveland Clinic Foundation, a residency in Internal Medicine with Baylor College of Medicine and a fellowship in Cardiology with University of California, San Francisco (“UCSF”). He received his M.D. from the University of Texas Southwestern Medical School.
As one of our co-founders, Dr. Agah has a deep understanding of our therapy platform, our history and culture. His initial education in biomedical engineering and vascular biology established the foundation for our therapy. His experience as a practicing vascular specialist and as the proctor for many of our procedures, has given him a deep understanding of the procedural aspects of our therapy. This background, taken together with his deep understanding of the cancers we are seeking to treat and his clinical trial experience enables him to bring a unique perspective and provide strong leadership to our Board.
Kirsten Angela Macfarlane. Ms. Macfarlane has served as our director since September 2018. She currently serves as CEO of Perceive Biotherapeutics, Inc., a biotech company dedicated to solving the major unsolved causes of vision loss. Ms. Macfarlane founded and serves as a Managing Partner of ForSight Labs, LLC an ophthalmic incubator formed in 2005 to focus on innovation in ophthalmology which has started eight companies in both therapeutics and medical devices. Ms. Macfarlane served as both the founding CEO of ForSight VISION4, Inc. through the acquisition (acquired by ROCHE - FDA approved SUSVIMO®), and the founding CEO of ForSight VISION5, Inc. and then at acquisition (acquired by Allergan plc). Previously, Ms. Macfarlane served as Chief Technology Counsel to The Foundry, LLC, a medical technology incubator, and Technology Counsel for Thomas J. Fogarty, M.D., a renowned physician/entrepreneur where she participated in formation development of nine companies from 1999 to 2004. She previously served on the senior management teams and counsel at TransVascular, Inc. (acquired by Medtronic), AneuRx, Inc. (acquired by Medtronic), and VidaMed Inc. (through its initial public offering). Ms. Macfarlane is an inventor on 25 U.S. issued patents. She received her BA in Business Administration from San Francisco State University, and her J.D. from Golden Gate University School of Law. She currently serves on the board of Perceive Biotherapeutics, Inc., ForSight VISION6, Inc., Recognify Life Sciences, Inc., Spiral Therapeutics, Inc., and the non-profit Fogarty Innovation and is a mentor in the Ferolyn Powell Leadership Program.
Ms. Macfarlane brings extensive operational, business development and management expertise to our Board. She has extensive experience in drug/device combination therapies with successful exits to large biotech and pharmaceutical companies. Her experience leading and growing companies is invaluable to us as we continue to develop our pipeline and identify new indications and small molecules to be used with our therapy platform. Ms. Macfarlane’s background and experience positions her well to serve as the Chairperson of the Compensation Committee and as a member of the Nominating and Corporate Governance Committee.
Laurence J. Marton, M.D. Dr. Marton serves as a consultant to industry and to nonprofit, government, and academic institutions and has served as a director of our company since 2012. In the nonprofit sector, Dr. Marton is an Emeritus Member of the Board of Trustees of the American Association for Cancer Research Foundation and is on the Board of Directors of Cancer Commons. In the for-profit sector, he serves on the Boards of Directors of Cellsonics, Matternet, Microsonic Systems, Nanotics, Omniox, and xCures and is an advisor to Assurance Health Data, PharmaJet, and the Precision Medicine World Conference. Previously, Dr. Marton was Dean of the University of Wisconsin Medical School and chaired the Department of Laboratory Medicine at UCSF, where he was a Professor in the Departments of Laboratory Medicine and Neurological Surgery. Dr. Marton received his M.D. from the Albert Einstein College of Medicine.
Dr. Marton brings deep experience in cancer research and treatment, operational expertise and years of working in the life sciences industry to our Board. His strategic advisory experience and membership on boards of various organizations make him a valuable member of our Audit and Nominating and Corporate Governance Committees.
| 100 |
Una S. Ryan, O.B.E., Ph.D., D.Sc. Dr. Ryan has served as our director since 2013. Dr. Ryan has extensive experience leading public, private and non-profit companies. She serves on the boards of Cortexyme, Inc. (Nasdaq: CRTX), Elemental Machines, Inc. (chair), Cambridge in America, and Bristol University U.S. Foundation (chair). Dr. Ryan is a limited partner at Breakout Ventures, L.P. and Lionheart Ventures Fund, L.P. She focuses on women-led ventures as Managing Director of Golden Seeds, and a partner in Astia Angels. She has a large portfolio of early-stage companies in San Francisco and Boston. She was a serial CEO of Diagnostics for All, Inc., Waltham Technologies, Inc., and AVANT Immunotherapeutics, Inc. (now Celldex Therapeutics, Inc.). Continuing a career translating science to successful businesses, Dr. Ryan is now translating science to art and founded ULUX, bringing science perspectives to the art world. Dr. Ryan holds a Ph.D. from Cambridge University, B.Sc. degrees and honorary D.Sc. from Bristol University. Her academic career included Professorships of Medicine at Miami, Washington and Boston Universities. She held the titles Howard Hughes Investigator, American Heart Association Established Investigator, and NIH MERIT Awardee. She has received numerous awards including the Albert Einstein Award (2007) for outstanding achievement in the life sciences, the Cartier Award (2009) and World Economic Forum Tech Pioneer (2011). In 2002, Her Majesty Queen Elizabeth II awarded Dr. Ryan the Order of the British Empire.
Dr. Ryan brings both extensive operations, investment and life sciences industry experience to our Board. Her public company operational, financial and corporate governance experience is a valuable resource to our Board and makes her well-positioned to serve as the Chair of the Nominating and Governance Committee and the Audit Committee.
Robert Spiegel, M.D., FACP. Dr. Spiegel brings more than 40 years of biopharmaceutical experience to our Board. He was involved in more than 30 successful New Drug Application (NDA) approvals by the FDA and the development and launch of multiple products with annual sales exceeding $1 billion. While at Schering-Plough, he served as Sr. Vice President of Worldwide Clinical Research and Chief Medical Officer. He was involved in the development of numerous cancer drugs and led the development of Remicade® (infliximab), Temodar® (temozolomide), and alpha-interferon (Intron A) through PH I-III studies, securing the first FDA approval for a biologic protein. After Merck acquired Schering-Plough in 2009, Dr. Spiegel became Chief Medical Officer at PTC Therapeutics where he led the company to EU Conditional Approval for the first drug ever approved for Duchene Muscular Dystrophy.
Dr. Spiegel currently serves on numerous publicly listed and privately held boards, including Geron Corporation, Cyclacel Pharmaceuticals, Ayala Pharmaceuticals, and Sun Pharma Advanced Research Corp. He also is an Associate Professor of Medicine at Weill Cornell Medical College, an Advisor to Warburg Pincus and Israel Biotech Fund, and a member of the Leukemia and Lymphoma Society TAP committee. Dr. Spiegel completed his Medical Oncology fellowship at the National Cancer Institute and received his M.D. from the University of Pennsylvania.
Dr. Spiegel brings to the Board valuable drug development expertise. His strategic advisory experience and membership on boards of various organizations would make him a valuable member of our Nominating and Corporate Governance Committee and Compensation Committee.
Other Officers
Ronald B. Kocak, CPA, CGMA. Mr. Kocak was appointed as our Principal Accounting Officer on February 8, 2024, and has served as our Vice President and Controller since October 2021. Prior to joining our company, from 2017 to 2020, he served as Controller and Senior Director of Finance at Sensei Biotherapeutics, Inc., where he led the finance and accounting activities, including the information technology operations, for initial public offering readiness. From 2008 through 2013, he served as the Corporate Controller and Chief Accounting Officer for Nabi Biopharmaceuticals, a Nasdaq-listed company, and was an integral member leading that company’s reverse merger acquisition and transitioning all finance and accounting activities. During his career, he has held various senior accounting and finance roles and provided consulting services for public and private companies. He holds a Bachelor of Science in accounting from Duquesne University and a Certified Public Accounting license in the Commonwealth of Virginia. Mr. Kocak is also a member of the American Institute of Certified Public Accountants and Association of Bioscience Financial Officers, and a Chartered Global Management Accountant. We believe Mr. Kocak is qualified for his position because of his extensive experience in public company accounting and finance operations and because of his existing knowledge of our company and our industry.
| 101 |
Leesa Gentry. Ms. Gentry has served as our Chief Clinical Officer since March 1, 2024 and previously served as our Senior Vice President of Clinical Operations since April 2023. From January 2019 to February 2023, Ms. Gentry served as Senior Vice President of Clinical Development and Operations for Evotec, GmbH (“Evotec”). Prior to her tenure at Evotec, Ms. Gentry spent 13 years, from November 2005 through December 2018, serving in positions of increasing responsibility for Otsuka Pharmaceutical Development and Commercialization. These positions include Director, Associate Director, Sr. Manager of Clinical Management, and Program Manager. Prior to her tenure with Otsuka, Ms. Gentry held clinical management positions within contract research organizations from 1995 to 2005, including IQVIA, PPD, Omnicare CR, and IBRD-Rostrum Global. Ms. Gentry holds an M.A. in psychology from the University of Mary Hardin-Baylor, as well as an M.S. in social gerontology and a B.A. in psychology from the University of Central Missouri. We believe Ms. Gentry is qualified for her position because of her extensive experience in clinical and regulatory affairs and because of her existing knowledge of our company and our industry.
Involvement in Certain Legal Proceedings
During the past ten years, none of our directors or executive officers have been involved in any legal proceedings described in Item 401(f) of Regulation S-K.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires our directors, executive officers, and persons who own more than 10% of a registered class of our equity securities, to file with the SEC reports of beneficial ownership and reports of changes in beneficial ownership in our securities. Based solely upon a review of Forms 3, 4 and 5, and amendments thereto, filed electronically with the SEC during the year ended December 31, 2023, we believe that all Section 16(a) filings applicable to its directors, officers, and 10% stockholders were filed on a timely basis during the year ended December 31, 2023, except that Angela Helms, our former Chief Operating Officer who resigned effective March 1, 2024, Ramtin Agah, our Chief Medical Officer and Chairman of the Board, and Shaun Bagai, our Chief Executive Officer and Director, each filed one late Form 4.
Board Leadership Structure
Our Board of Directors is currently chaired by Dr. Agah. We separate the roles of Chief Executive Officer and Chairman of the Board in recognition of the differences between the two roles. The Chief Executive Officer is responsible for setting the strategic direction for our company and the day-to-day leadership and performance of our company, while the Chairman of the Board of Directors provides guidance to the Chief Executive Officer and presides over meetings of the full Board of Directors. We believe that this separation of responsibilities provides a balanced approach to managing the Board of Directors and overseeing our company.
Our Board of Directors had appointed a Lead Independent Director, David Diamond, who passed away in late 2023. At the present time, given the attributes of our independent directors, our Board of Directors does not believe a Lead Independent Director is necessary.
Role of the Board in Risk Oversight
Our Board of Directors takes an active role, as a whole and also at the committee level, in overseeing the management of our risks. Our Board of Directors is responsible for general oversight of risks and regularly reviews and discusses with management our major risk exposures, their potential impact on our business and the steps we take to manage them. The risk oversight process includes receiving regular reports from committees of the Board and members of senior management to enable our Board of Directors to understand our risk identification, risk management and risk mitigation strategies with respect to areas of potential material risk, including operations, clinical, finance, legal, regulatory, strategic and reputational risk.
The Compensation Committee is responsible for overseeing the management of risks relating to our executive compensation plans and arrangements. The Audit Committee is responsible for overseeing the management of risks relating to accounting matters and financial reporting. The Nominating and Corporate Governance Committee is responsible for overseeing the management of risks associated with the independence of our Board of Directors and potential conflicts of interest. Although each committee is responsible for evaluating certain risks and overseeing the management of such risks, our entire Board of Directors is regularly informed of each committee’s risk oversight activities through regular reports to the Board. The Board’s role in risk oversight is consistent with our leadership structure, with the Chief Executive Officer and other members of senior management having responsibility for assessing and managing our risk exposure, and the Board and its committees providing oversight in connection with those efforts.
| 102 |
Executive Sessions of Independent Directors
In order to promote open discussion among independent directors, our Board of Directors has a policy of conducting executive sessions of independent directors on a periodic basis, and typically has such a session after each regularly scheduled Board of Directors meeting.
Board Meetings
During the fiscal year ended December 31, 2023, our Board of Directors held six meetings (including regularly scheduled and special meetings), and each director attended at least 75% of the aggregate of (i) the total number of meetings of our Board of Directors held during the period for which he or she has been a director and (ii) the total number of meetings held by all committees of our Board of Directors on which he or she served during the periods that he or she served. Although we do not have a formal policy regarding attendance by members of our Board of Directors at annual meetings of stockholders, we encourage our directors to attend.
Board Committees
Our Board of Directors has established an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee, each of which has the composition and the responsibilities described below.
As of the date of this Report, the membership of the standing committees was as follows:
| Board Member | Audit | Compensation | Nominating & Corporate Governance | |||
| Shaun R. Bagai | — | — | — | |||
| Ramtin Agah, M.D. | — | — | — | |||
| Kirsten Angela Macfarlane | — | Chair | X | |||
| Laurence J. Marton, M.D. | X | — | X | |||
| Una S. Ryan, O.B.E., Ph.D., D.Sc. | Chair | — | Chair | |||
| Robert J. Spiegel, M.D. FACP | X | X | — |
Audit Committee
The members of our Audit Committee are Una S. Ryan, O.B.E., Ph.D., D.Sc., Laurence J. Marton, M.D. and Robert J. Spiegel, M.D. FACP. Dr. Ryan is the chair of our Audit Committee. Our Board has determined that our independent Director Dr. Ryan qualifies as an “audit committee financial expert,” as that term is defined under the SEC rules implementing Section 407 of the Sarbanes-Oxley Act of 2002, who possesses financial sophistication, as defined under the rules of Nasdaq. Our Audit Committee oversees our corporate accounting and financial reporting process and assists our Board of Directors in monitoring our financial systems. As more fully described in its charter, the Audit Committee also:
| ● | selects, retains and evaluates the performance and independence of our independent registered public accounting firm; | |
| ● | approves audit and non-audit services and fees of the independent registered public accounting firm; | |
| ● | reviews financial statements and discusses with management and the independent registered public accounting firm our annual audited and quarterly financial statements, the results of the independent audit and the quarterly reviews and the reports and certifications regarding internal controls over financial reporting and disclosure controls; |
| 103 |
| ● | prepares the Audit Committee report that the SEC requires to be included in our annual proxy statement; | |
| ● | reviews reports and communications from the independent registered public accounting firm; | |
| ● | reviews the adequacy and effectiveness of our internal controls and disclosure controls and procedures; | |
| ● | reviews our policies on risk assessment and risk management; | |
| ● | monitors and administers our Code of Business Conduct and Ethics; | |
| ● | reviews, approves and oversees related party transactions and any other potential conflict of interest situations; and | |
| ● | establishes and oversees procedures for the receipt, retention, and treatment of accounting-related complaints and the confidential submission by our employees of concerns regarding questionable accounting or auditing matters. |
Our Audit Committee operates under a written charter that satisfies the applicable rules of the SEC and the listing standards of Nasdaq. A current copy of the charter is available on our website under the “Investors - Corporate Governance – Documents and Charters” tabs at https://renovorx.com/investors/corporate-governance/documents-charters. During 2023, our Audit Committee held 4 meetings.
Compensation Committee
The members of our Compensation Committee are Kirsten Angela Macfarlane and Robert J. Spiegel, M.D. FACP. Ms. Macfarlane is the chair of our Compensation Committee. Our Compensation Committee oversees our overall compensation philosophy and compensation policies, incentive- and equity-based plans and policies and evaluates and approves the compensation plans and programs advisable for our company. As more fully described in its charter, the Compensation Committee also:
| ● | reviews and approves, or recommends to the Board of Directors for approval, corporate performance goals and objectives; | |
| ● | reviews and approves or recommends to the Board of Directors for approval compensation for our Chief Executive Officer, other executive officers and directors; | |
| ● | reviews and approves the adoption, amendment and termination of any employment and any severance, change in control or other compensatory arrangements for our executive officers; | |
| ● | reviews regional and industry-wide compensation practices and trends to assess the propriety, adequacy and competitiveness of our executive compensation programs; | |
| ● | reviews our compensation policies and practices for all employees regarding risk-taking incentives and risk management policies and practices; and | |
| ● | administers our equity compensation plans. |
Our Compensation Committee operates under a written charter that satisfies the applicable rules of the SEC and the listing standards of Nasdaq. A current copy of the charter is available on our website under the “Investors - Corporate Governance – Documents and Charters” tabs at https://renovorx.com/investors/corporate-governance/documents-charters. During 2023, our Compensation Committee held 5 meetings.
| 104 |
Compensation Committee Interlocks and Inside Participation
None of the members of our Compensation Committee is or has been an officer or employee of our company. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the Board of Directors or Compensation Committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire Board of Directors) of any entity that has one or more executive officers serving on our Board of Directors or Compensation Committee.
Nominating and Corporate Governance Committee
The members of our Nominating and Corporate Governance Committee are Una S. Ryan, O.B.E., Ph.D., D.Sc., Laurence J. Marton, M.D., and Kirsten Angela Macfarlane. Dr. Ryan is the chair of our Nominating and Corporate Governance Committee. Our Nominating and Corporate Governance Committee oversees and assists our Board of Directors in identifying, evaluating, and recommending nominees for election to our Board of Directors and its committees, based on its evaluation of the Board’s and its committees’ composition, reviewing developments in corporate governance practices and maintaining our corporate governance policies. As more fully described in its charter, the Nominating and Corporate Governance Committee also:
| ● | evaluates the adequacy of our corporate governance practices and reporting; | |
| ● | evaluates the performance of our Board of Directors and of individual directors; | |
| ● | reviews our Code of Business Conduct and Ethics and recommends changes to our Board of Directors, as appropriate; and | |
| ● | reviews stockholder proposals and makes recommendations to our Board of Directors, as appropriate. |
Our Nominating and Corporate Governance Committee operates under a written charter that satisfies the applicable rules of the SEC and the listing standards of Nasdaq. A current copy of the charter is available on our website under the “Investors - Corporate Governance – Documents and Charters” tabs at https://renovorx.com/investors/corporate-governance/documents-charters. Our Nominating and Corporate Governance Committee was constituted in June 2021. During 2023, our Nominating and Corporate Governance Committee held 3 meetings.
Director Nomination Process
The Nominating and Corporate Governance Committee is responsible for identifying individuals qualified to become members of the Board, consistent with criteria approved by the Board, and for recommending to the Board the nominees for election as directors at any meeting of stockholders and the persons to be elected by the Board to fill vacancies or newly created directorships. The Nominating and Corporate Governance Committee periodically reviews with the Board the size and composition of the Board as a whole and the requisite skills and criteria for new directors.
Review of Incumbent Directors
The Nominating and Corporate Governance Committee considers, among other things, the performance, experience, tenure, qualifications, and skills of incumbent directors, including their respective attendance records and personal attributes as required under our Corporate Governance Guidelines, as well as the need for diversity and financial or other specialized expertise, before making a determination to recommend incumbent directors for nomination for election or re-election as directors. The Nominating and Corporate Governance Committee generally will nominate incumbent directors who continue to qualify based upon the criteria considered by the Committee and who are willing to continue to serve on the Board.
| 105 |
Stockholder Recommendations of Director Candidates
Our Nominating and Corporate Governance Committee will consider candidates for director recommended by stockholders, so long as such recommendations comply with the requirements set forth in our Bylaws and applicable SEC rules and regulations.
We do not have a formal policy regarding consideration of director candidate recommendations from our stockholders. However, the Nominating and Corporate Governance Committee will evaluate nominees recommended by stockholders in the same manner as it evaluates other nominees.
Criteria for Nomination to the Board
In evaluating prospective candidates, the Nominating and Corporate Governance Committee takes into account the criteria established by the Board in our Corporate Governance Guidelines and other factors it considers appropriate, including but not limited to the characteristics of independence, diversity, age, skills and experience, the needs and composition of the Board as a whole (including diversity of skills, background and experience), our operating requirements, the performance and continued tenure of incumbent directors, the balance of management and independent directors and the need for financial or other specialized expertise. In accordance with our Corporate Governance Guidelines, the Board believes that candidates for director should have certain minimum qualifications, including being able to read and understand basic financial statements, being over 21 years of age and having the highest personal integrity and ethics.
Pursuant to our Corporate Governance Guidelines, the Nominating and Corporate Governance Committee will also review director candidates’ personal attributes in accordance with the following general criteria:
| ● | nominees should possess relevant expertise upon which to be able to offer advice and guidance to management; | |
| ● | nominees should have sufficient time to devote to our affairs; | |
| ● | nominees should have demonstrated excellence in his/her field; and | |
| ● | nominees should have the ability to exercise sound business judgment and have the commitment to represent the long-term interests of our stockholders. |
Although our Board of Directors does not maintain a specific policy with respect to board diversity, it believes that our Board of Directors should be a diverse body, and our Nominating and Corporate Governance Committee may consider such factors as differences in professional background, education, skill, and other individual qualities and attributes that contribute to the total mix of viewpoints and experience represented on our Board of Directors.
Process for Identifying and Evaluating Nominees
Candidates for director may come to the attention of the Nominating and Corporate Governance Committee through current members of the Board, professional search firms, stockholders or industry sources. The Nominating and Corporate Governance Committee first evaluates director candidates by reviewing their biographical information and qualifications. Potentially qualified candidates will meet with our Chief Executive Officer and at least one member of the Nominating and Corporate Governance Committee and then, if appropriate, with other members of the Committee and the Board. After completing the evaluation and interviews and checking the candidates’ references, the Nominating and Corporate Governance Committee determines which individuals are qualified to become Board members and makes a recommendation to the Board as to the individuals who should be nominated for election by the stockholders at a meeting or elected by the Board to fill a vacancy or newly created directorship. The Board makes the final determination whether to nominate or elect a candidate after considering the Nominating and Corporate Governance Committee’s recommendation.
Board Diversity
Our directors represent a wide variety of skills and backgrounds. The below Board Diversity Matrix reports self-identified diversity statistics for the Board in the format required by the Nasdaq Marketplace Rules. As demonstrated by the below Board Diversity Matrix, we are compliant with the diversity requirements of Nasdaq Listing Rule 5605(f) and the California Corporations Code.
| 106 |
| Board Diversity Matrix | ||||||||||||||||
| Total Number of Directors | 6 | |||||||||||||||
| Female | Male | Non-Binary | Did Not Disclose Gender |
|||||||||||||
| Part I: Gender Identity | ||||||||||||||||
| Directors | 2 | 4 | 0 | 0 | ||||||||||||
| Part II: Demographic Background | ||||||||||||||||
| African American or Black | 0 | 0 | 0 | 0 | ||||||||||||
| Alaskan Native or Native American | 0 | 0 | 0 | 0 | ||||||||||||
| Asian | 0 | 1 | 0 | 0 | ||||||||||||
| Hispanic or Latinx | 0 | 0 | 0 | 0 | ||||||||||||
| Native Hawaiian or Pacific Islander | 0 | 0 | 0 | 0 | ||||||||||||
| White | 1 | 2 | 0 | 0 | ||||||||||||
| Two or more races or ethnicities | 1 | 0 | 0 | 0 | ||||||||||||
| LGBTQ+ | 0 | 0 | 0 | 0 | ||||||||||||
| Did not disclose demographic background | 1 | 0 | 0 | 0 | ||||||||||||
Insider Trading Policy
On September 7, 2023, we adopted an amended and restated insider trading policy and procedures governing the purchase, sale, and/or other dispositions of our securities by directors, officers and employees, which are reasonably designed to promote compliance with insider trading laws, rules and regulations, and applicable Nasdaq listing standards (the “Insider Trading Policy”).
The foregoing description of the Insider Trading Policy does not purport to be complete and is qualified in its entirety by the terms and conditions of the Insider Trading Policy, a copy of which is filed as an exhibit to this Report and is incorporated herein by reference.
Corporate Governance Guidelines and Code of Business Conduct and Ethics
Our Board of Directors has adopted Corporate Governance Guidelines that address items such as the qualifications and responsibilities of our directors and director candidates and corporate governance policies and standards applicable to us in general. In addition, our Board of Directors has adopted a Code of Business Conduct and Ethics that applies to all of our employees, officers and directors, including our Chief Executive Officer and other executive and senior financial officers. The full text of our Corporate Governance Guidelines and our Code of Business Conduct and Ethics is available on our website under the “Investors - Corporate Governance – Documents and Charters” tabs https://renovorx.com/investors/corporate-governance/documents-charters. We will post amendments to our Code of Business Conduct and Ethics or waivers of our Code of Business Conduct and Ethics for directors and executive officers on the same website.
Item 11. EXECUTIVE COMPENSATION
This section describes the 2023 compensation program established by the Compensation Committee for our named executive officers (“NEOs”). Our NEOs, consisting of our principal executive officer and the two most highly compensated executive officers (other than our principal executive officer) who were serving as executive officers as of December 31, 2023 were:
| ● | Shaun R. Bagai, Chief Executive Officer | |
| ● | Ramtin Agah, M.D., Chief Medical Officer | |
| ● | Angela Gill Nelms, Chief Operating Officer (note Ms. Nelms resigned her position on February 16, 2024) |
| 107 |
Processes and Procedures for Compensation Decisions
Our Compensation Committee is responsible for the executive compensation programs for our executive officers and reports to our Board of Directors on its discussions, decisions and other actions. Typically, our Chief Executive Officer makes recommendations to our Compensation Committee, often attends committee meetings and is involved in the determination of compensation for the respective executive officers who report to him, except that our Chief Executive Officer does not make recommendations as to his own compensation. Our Chief Executive Officer makes recommendations to our Compensation Committee regarding short- and long-term compensation for all executive officers (other than himself) based on our results, an individual executive officer’s contribution toward these results and performance toward individual goal achievement. Our Compensation Committee then reviews the recommendations and other data. Our Compensation Committee makes decisions as to total compensation for each executive officer, although it may instead, in its discretion, make recommendations to our Board of Directors regarding executive compensation.
Our Compensation Committee is authorized to retain the services of one or more executive compensation advisors, as it sees fit, in connection with the establishment of our compensation programs and related policies. In 2022, our Compensation Committee engaged Compensia, Inc. (“Compensia”), a third-party compensation consultant, to provide it with information, recommendations and other advice relating to executive compensation on an ongoing basis. Accordingly, Compensia serves at the discretion of our Compensation Committee. Our Compensation Committee engaged Compensia to assist in developing a group of peer companies to help us determine the appropriate level of overall compensation for our executive officers, as well as assess each separate element of compensation, with a goal of promoting executive compensation that is competitive and fair.
In connection with Compensia’s engagement, our Compensation Committee conducted such a review and concluded that it was not aware of any conflict of interest that had been raised by work performed by Compensia or the individual consultants employed by Compensia that perform services for our Compensation Committee.
Summary Compensation Table
The following table sets forth information concerning the compensation of our NEOs for the years ended December 31, 2023 and 2022
| Name and Position | Year | Salary(1) ($) | Bonus(2) ($) | Option Awards(3) ($) | Nonequity Incentive Plan Compensation(4) ($) | Other Compensation ($) | Total ($) | ||||||||||||||||||||
| Shaun R. Bagai | 2023 | 520,000 | 69,376 | 349,739 | — | — | 936,779 | ||||||||||||||||||||
| Chief Executive Officer | 2022 | 495,000 | — | 123,324 | 222,750 | — | 841,074 | ||||||||||||||||||||
| Ramtin Agah, M.D. | 2023 | — | 29,444 | 174,870 | — | 303,450 | 506,772 | ||||||||||||||||||||
| Chief Medical Officer | 2022 | — | — | 54,621 | 91,035 | 289,000 | 434,655 | ||||||||||||||||||||
| Angela Gill Nelms | 2023 | 412,000 | 34,980 | 87,435 | — | 14,397 | (6) | 547,634 | |||||||||||||||||||
| Chief Operating Officer | 2022 | 115,152 | — | 535,736 | 35,901 | (5) | 20,500 | (7) | 707,288 | ||||||||||||||||||
| (1) | Represents annual salary for Mr. Bagai and Ms. Nelms and annual consulting fee for Dr. Agah. |
| (2) | The amount represents 50% of the cash bonus earned by each named executive officers in recognition of the company and individual performance during the fiscal year ended December 31, 2023, each NEOs was awarded fully vested non-qualified stock options with a 10-year term. The amounts in this column represent the aggregate grant date fair value of the stock option awards computed in accordance with FASB guidance on stock-based compensation. The grant date fair value of stock options is determined based on the number of options awarded and the fair value of the stock option on the grant date, which is the Black Scholes value of closing sales price per share of our common stock. |
| (3) | The amounts in this column represent the aggregate grant date fair value of the stock option awards computed in accordance with FASB guidance on stock-based compensation. The relevant assumptions made in the valuations for the 2023 and 2022 stock option awards may be found in Note 7 of the Notes to the Financial Statements in our Report. The grant date fair value of stock options is determined based on the number of options awarded and the fair value of the stock option on the grant date, which is the Black Scholes value of closing sales price per share of our common stock. |
| (4) | The amounts in this column represent the annual cash bonuses earned by each of the named executive officers in recognition of company and individual performance during the fiscal year ended December 31, 2022 under our 2022 Bonus Plan and are pro-rated based on length of service with us. |
| (5) | Represents a bonus payment of $35,901 paid in February 2023 to Ms. Nelms in recognition of company and individual performance during the year ended December 31, 2023 pursuant to the 2022 Bonus Plan. |
| (6) | Represents a matching contribution under our 401(k) plan of $13,200 and fringe benefit supplement of $1,197. |
| (7) | Represents a relocation assistance allowance grossed up for taxes in a total amount of $20,000 and matching contributions under our 401(k) plan of $500. |
| 108 |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information with respect to outstanding equity awards for each of our NEOs as of December 31, 2023.
| Option Awards | ||||||||||||||||
| Name | Grant Date | Number of Shares of Stock Underlying Unexercised Options Exercisable | Number of Shares of Stock Underlying Unexercised Options Unexercisable | Option Exercise Price | Option Expiration Date | |||||||||||
| Shaun R. Bagai | 03/19/2017 | 60,000 | — | $ | 0.50 | 05/18/2027 | ||||||||||
| 07/11/2018 | 120,000 | — | $ | 0.65 | 07/10/2028 | |||||||||||
| 09/30/2021 | 68,755 | 49,111 | (1) | $ | 6.04 | 09/29/2031 | ||||||||||
| 03/10/2022 | 28,182 | 20,131 | (1) | $ | 3.17 | 03/10/2032 | ||||||||||
| 03/15/2023 | 32,500 | 97,500 | (2) | $ | 3.29 | 03/15/2033 | ||||||||||
| Ramtin Agah, M.D. | 05/19/2017 | 60,000 | — | $ | 0.50 | 05/18/2027 | ||||||||||
| 07/11/2018 | 40,000 | — | $ | 0.65 | 07/10/2028 | |||||||||||
| 06/04/2021 | 20,000 | — | $ | 2.45 | 06/03/2031 | |||||||||||
| 09/30/2021 | 30,451 | 21,752 | (1) | $ | 6.04 | 09/29/2031 | ||||||||||
| 03/10/2022 | 12,482 | 8,916 | (1) | $ | 3.17 | 03/10/2032 | ||||||||||
| 03/15/2023 | 16,250 | 48,750 | (2) | $ | 3.29 | 03/15/2033 | ||||||||||
| Angela Gill Nelms | 09/19/2022 | 85,696, | 188,534 | (3) | $ | 2.42 | 09/19/2032 | |||||||||
| 03/15/2023 | 8,125 | 24,375 | (2) | $ | 3.29 | 03/15/2033 | ||||||||||
| (1) | The stock option vests monthly over four years commencing on September 26, 2021, subject to continued service with us through the applicable vesting date. |
| (2) | The stock option vests monthly over four years commencing on January 1, 2023, subject to continued service with us through the applicable vesting date. |
| (3) | The stock option vests over four years, with 25% vesting on September 19, 2023, the first anniversary of the vesting commencement date, and the remainder vesting monthly thereafter, subject to continued service with us through the applicable vesting date. |
Narrative Disclosure to Summary Compensation Table
The primary elements of compensation for our NEOs are base salary, annual performance bonuses and equity awards. The NEOs also participate in employee benefit plans and programs that we offer to our other employees, as described below.
Annual Base Salary
We pay our NEOs a base salary or base consulting fee to compensate them for the satisfactory performance of services rendered to us. The base compensation payable to each NEO is intended to provide a fixed component of compensation reflecting the executive’s skill set, experience, role and responsibilities.
Our Compensation Committee will periodically review the base salaries of our NEOs and will recommend adjustments as necessary to maintain base compensation at competitive levels.
As of January 2023, Mr. Bagai’s annual base salary was increased to $520,000, to align Mr. Bagai’s compensation more closely to the 50th percentile of the peer group of companies that we benchmark our compensation against. As of January 2024, Mr. Bagai’s annual base salary decreased 25%, effective February 1, 2024, to $390,000. As of January 2023, Dr. Agah’s monthly consulting fee decreased to $25,287.50, based on Dr. Agah spending no less than 24 hours per week on our matters. As of January 2024, Dr. Agah’s monthly consulting fee decreased 25%, effective February 1, 2024, to $18,965.63. We may, in our discretion, proportionally adjust the monthly consulting fee if Dr. Agah’s time commitment decreases. As of January 2023, Ms. Nelms annual base salary was increased to $412,000. As of January 2024, Ms. Nelms’ annual base salary decreased 25%, effective February 1, 2024, to $309,000.
| 109 |
Equity-Based Incentive Awards
Our equity-based incentive awards are designed to align our interests and the interests of our stockholders with those of our employees and consultants, including our NEOs. In general, the Board or its Compensation Committee is responsible for approving equity grants. Following our IPO, we generally grant equity awards to our employees, including our NEOs, as long-term incentive components of our compensation program. We typically grant equity awards to new hires upon their commencing employment with us. Additionally, we may grant equity awards at such times as the Board determines appropriate. Generally, our equity awards vest over four years, subject to the employee’s continued employment with us on each vesting date.
In February 2022, our 2021 Omnibus Equity Incentive Plan (as amended, the “2021 Plan”) was amended and restated to reflect various changes, including removal of vesting restrictions within one year from the date of grant for at least ninety-five percent (95%) of the awards, and upon a Change of Control (as defined in the 2021 Plan), the administrator of the 2021 Plan may provide that the successor corporation may assume or substitute any portion of an award under the 2021 Plan.
In March 2023, we granted Mr. Bagai an option to purchase 130,000 shares of our common stock under our 2021 Plan. The option vests monthly over a 48-month period and has an exercise price of $3.29, which was the fair market value of our common stock on the grant date.
In March 2023, we granted Dr. Agah an option to purchase an additional 65,000 shares of our common stock under the 2021 Plan. The option vests monthly over a 48-month period and has an exercise price of $3.29, which was the fair market value of our common stock on the grant date.
In March 2023, we granted Ms. Nelms an option to purchase an additional 32,500 shares of our common stock under our 2021 Plan. The option vests monthly over a 48-month period and has an exercise price of $3.29, which was the fair market value of our common stock on the grant date.
Employment Arrangements with our Named Executive Officers
Shaun R. Bagai
Mr. Bagai has been our Chief Executive Officer since 2014, initially as a consultant. In December 2015, we entered into an offer letter with Mr. Bagai that set forth the terms and conditions of his employment. The letter became effective on January 1, 2016, and was amended in June 2017 and December 2020. The amended offer letter provided for (i) an annual base salary of $300,000; (ii) a $2,500 monthly stipend for Mr. Bagai’s health insurance expenses, which continued until we begin offering health insurance as a benefit to all employees; (iii) a $70,000 bonus to be paid in connection with the IPO; and (iv) an additional performance bonus upon achievement of certain clinical and company milestones established by the Board in 2021. Upon consummation of our IPO, Mr. Bagai’s annual base salary was increased to $363,000.
In November 2021, we entered into a Confirmatory Employment Letter with Mr. Bagai. The Confirmatory Employment Letter has no specific term and provides that Mr. Bagai is an at-will employee. The Confirmatory Employment Letter supersedes all existing agreements and understandings that Mr. Bagai may have entered into concerning his employment relationship with us. In March 2022, Mr. Bagai’s annual base salary was increased to $495,000 from $363,000, retroactively effective to January 1, 2022. Mr. Bagai is eligible for an annual target cash incentive bonus equal to 50% of his annual base salary and will also be entitled to receive other employee benefits generally available to all of our employees. In November 2021, we entered into a Change in Control and Severance Agreement with Mr. Bagai, the terms of which are described below under the heading “Change in Control and Severance Agreement.”
| 110 |
Ramtin Agah, M.D.
In January 2018, we entered into a consulting agreement with Dr. Agah, pursuant to which Dr. Agah provides consulting services as our Chief Medical Officer by overseeing our sponsored clinical trials. The Agreement continues in force for as long as Dr. Agah is providing consulting services and may be terminated by either party on thirty (30) days’ notice. Initially, the sole compensation payable to Dr. Agah was the continued vesting of his options to purchase shares of common stock. In December 2018, Dr. Agah’s agreement was amended to provide that he would receive cash compensation of $4,000 per month for certain proctoring services, and in September 2019, his compensation was increased to $10,000 per month to compensate for additional services he was providing. In November 2021, we entered into a third amendment to the Consulting Agreement with Dr. Agah which provides for a monthly consulting fee of $21,667.67, based on Dr. Agah spending no less than 24 hours per week on matters relating to our company. Dr. Agah’s monthly consulting fee was increased to $24,083.33 effective January 1, 2022. We may, in our discretion, proportionally adjust the monthly consulting fee if Dr. Agah’s time commitment decreases. Dr. Agah’s agreement also provides for his eligibility for an annual target cash incentive bonus equal to 35% of his annualized base consulting fee. In November 2021, we entered into a Change in Control and Severance Agreement with Dr. Agah, the terms of which are described below under the heading “Change in Control and Severance Agreement.”
Ronald B. Kocak
In February 2024, we entered an Amended and Restated Employment Letter with Mr. Kocak (the “Kocak Letter”). Pursuant to the Kocak Letter, we pay to Mr. Kocak an annual base salary of $235,000. We may, in our sole discretion, grant to Mr. Kocak an annual incentive bonus based upon targets set by the Board and its Compensation Committee. Mr. Kocak’s bonus target shall be up to 35% of his base salary. Pursuant to the Kocak Letter, we also granted to Mr. Kocak, effective February 8, 2024, an option to purchase 15,000 shares of our common stock with an exercise price of $1.56 per share. Such option shall vest over four years. 1/48th of the shares subject to such option will vest on each monthly anniversary of the vesting commencement date (February 9, 2024), in each case subject to Mr. Kocak’s continued service with us through the applicable vesting date. Such option and its vesting shall be subject to, and governed by, the terms and conditions of the 2021 Plan, except that the Kocak Letter provides that such option and all other options previously granted and to be granted to Mr. Kocak under the Plan will be subject to a “double trigger” vesting provision upon a Change in Control (as defined in the 2021Plan). Mr. Kocak’s employment is at will, meaning that either he or we may terminate the employment at any time for any reason or no reason. The Kocak Letter also contains customary provisions for confidentiality and matters related to intellectual property and company property.
Leesa Gentry
In March 2024, we entered an Amended and Restated Employment Letter with Ms. Gentry (the “Gentry Letter”). Pursuant to the Gentry Letter, we pay to Ms. Gentry an annual base salary of $325,000. We may, in our sole discretion, grant to Ms. Gentry an annual incentive bonus based upon targets set by the Board and its Compensation Committee. Ms. Gentry’s bonus target shall be up to 35% of her base salary. Pursuant to the Gentry Letter, we also granted to Ms. Gentry, effective March 6, 2024, an option to purchase 190,000 shares of our common stock with an exercise price of $1.69 per share. Such option shall vest over four years. 1/48th of the shares subject to such option will vest on each monthly anniversary of the vesting commencement date (March 1, 2024), in each case subject to Ms. Gentry’s continued service with us through the applicable vesting date. Such option and its vesting shall be subject to, and governed by, the terms and conditions of the 2021 Plan. Ms. Gentry’s employment is at will, meaning that either he or we may terminate the employment at any time for any reason or no reason. The Gentry Letter also contains customary provisions for confidentiality and matters related to intellectual property and company property.
Change in Control and Severance Agreements
In November 2021, we entered into a Change in Control and Severance Agreement, or the Severance Agreement, with each of Mr. Bagai and Dr. Agah and in August 2022, we entered into the Severance Agreement with Ms. Nelms (each an “Executive” and collectively, the “Executives”). Capitalized terms used herein and not otherwise defined shall have the meaning assigned to such term in the Severance Agreement. Each Severance Agreement will continue indefinitely until terminated by written consent of the parties to the Severance Agreement.
| 111 |
Termination Outside of Change in Control Period
Under the Severance Agreement, if Mr. Bagai, Dr. Agah or Ms. Nelms are terminated outside a period beginning on the date of a Change in Control and ending on (and inclusive of) the date that is the one-year anniversary of a Change in Control (the “Change in Control Period”), either by us without Cause (other than due to death or Disability) or by the Executive for Good Reason, they will receive:
| ● | Annual Base Compensation Severance: A single, lump sum payment equal to the specified percent of the Executive Officer’s Annual Base Compensation (which is base salary or, for Dr. Agah, annual base consulting fee, if applicable), which was in effect immediately before such termination (or, if the termination is due to a resignation for Good Reason based on a material reduction in the Executive Officer’s annual base salary (or, for Dr. Agah, annual base consulting fee, if applicable), then Executive’s annual base salary (or, for Dr. Agah, annual base consulting fee, if applicable) in effect immediately prior to the reduction), or if greater, the base salary (or, for Dr. Agah, annual base consulting fee, if applicable), in effect immediately prior to the Change in Control): |
| (i) | Mr. Bagai: 100% of Annual Base Compensation | |
| (ii) | Dr. Agah and Ms. Nelms: 50% of Annual Base Compensation; and |
| ● | Bonus Severance: A single, lump sum payment of the pro rata portion (based on period of employment) of the Executive Officer’s target bonus in effect immediately before such termination or if greater, the target bonus in effect immediately prior to the Change in Control; and | |
| ● | COBRA Severance: payment or reimbursement of COBRA continuation coverage premiums for group health, dental and vision coverage for the Executive Officer and his eligible dependents, for: |
| (i) | Mr. Bagai: up to 12 months | |
| (ii) | Dr. Agah and Ms. Nelms (if Dr. Agah was an employee immediately prior to the termination): up to 6 months |
Or, if providing such payment would violate applicable law, a taxable payment for an equivalent amount in lieu thereof.
Termination During Change in Control Period
Under the Severance Agreement, if Mr. Bagai, Dr. Agah or Ms. Nelms are terminated during the Change in Control Period, either by us without Cause (other than due to death or Disability), or by the Executive for Good Reason, they will receive:
| ● | Base Compensation Severance: A single, lump sum payment equal to the specified percent of the Executive Officer’s Annual Base Compensation: |
| (i) | Mr. Bagai: 100% of Annual Base Compensation | |
| (ii) | Dr. Agah and Ms. Nelms: 100% of Annual Base Compensation; and |
| ● | COBRA Severance: payment or reimbursement of COBRA continuation coverage premiums for group health, dental and vision coverage for the executive officer and his eligible dependents, for: |
| (i) | Mr. Bagai: up to 18 months | |
| (ii) | Dr. Agah and Ms. Nelms (if Dr. Agah was an employee immediately prior to the termination): up to 12 months |
Or, if providing such payment would violate applicable law, a taxable payment for an equivalent amount in lieu thereof; and
| ● | Vesting Acceleration of Service-based Equity Awards: Full vesting of the outstanding and unvested equity awards (other than equity award subject to performance-based vesting criteria). |
| 112 |
The Severance Agreement provides that if any payments or benefits received by Mr. Bagai, Dr. Agah or Ms. Nelms under the Severance Agreement or otherwise would constitute “parachute payments” within the meaning of Section 280G of the Internal Revenue Code (the “Code”) and be subject to excise taxes imposed by Section 4999 of the Code, such amount will either be delivered in full or reduced so as not to be subject to excise taxation, whichever amount is higher. The Severance Agreement does not require us to provide any tax gross-ups.
To receive the severance described above, the Executive Officers must sign and not revoke our standard separation agreement and release of claims within the timeframe that is set forth in the Severance Agreement.
Other Elements of Compensation
Perquisites, Health, Welfare and Retirement Benefits
Our NEOs are eligible to participate in our employee benefit plans, including our medical, dental and vision plans, in each case on the same basis as all of our other employees.
We generally do not provide perquisites or personal benefits to our NEOs, except in limited circumstances.
401(k) Plan
Effective January 2022, we established a defined contribution employee retirement plan, or 401(k) plan, for our employees. Our NEOs are eligible to participate in the 401(k) plan on the same basis as our other employees. The 401(k) plan is intended to qualify as a tax-qualified plan under Section 401(k) of the U.S. Internal Revenue Code of 1986, as amended (the Code). The 401(k) plan provides that each participant may make pre-tax deferrals from his or her compensation up to the statutory limit and other testing limits. Participants that are 50 years or older can also make “catch-up” contributions. Commencing in 2022, we will make matching contributions of 100% of employee contributions up to 4% of their eligible compensation. Participant contributions are held and invested, pursuant to the participant’s instructions, by the plan’s trustee.
Key Service Provider Incentive Compensation Plan
Our Key Service Provider Incentive Compensation Plan, or Bonus Plan allows the Compensation Committee to (i) determine which employees or other service providers may receive incentive awards under the Bonus Plan, and (ii) provide incentive awards to selected employees, including our NEOs and other service providers, which may be based upon performance goals established by our Compensation Committee. Our Compensation Committee, in its sole discretion, may establish a target award for each participant under the Bonus Plan, which may be expressed as a percentage of the participant’s average annual base salary for the applicable performance period or a fixed dollar amount or such other amount or based on such other formula or factors as the Compensation Committee determines.
Under the Bonus Plan, our Compensation Committee will determine the performance goals, if any, applicable to awards and such performance goals may differ from participant to participant and from award to award. Performance goals may be based on any factors our Compensation Committee determines relevant, including, without limitation, on an individual, divisional, portfolio, project, business unit, segment, or company-wide basis, and may include criteria related to research and development, regulatory, business development, financial and operational performance or other subjective or objective criteria. Any criteria used may be measured on such basis as our Compensation Committee determines. As determined by our Compensation Committee, the performance goals may be based on GAAP or non-GAAP results and any actual results may be adjusted by our Compensation Committee for one-time items or unbudgeted or unexpected items and/or payment of actual awards when determining whether the performance goals have been met.
| 113 |
Our Compensation Committee, at any time prior to payment of an actual award, may increase, reduce or eliminate a participant’s actual award, and/or increase, reduce or eliminate the amount allocated to the bonus pool. The actual award may be below, at or above a participant’s target award, in our Compensation Committee’s discretion. Our Compensation Committee may determine the amount of any increase, reduction or elimination of an actual award based on such factors as it deems relevant, and it will not be required to establish any allocation or weighting with respect to the factors it considers.
Actual awards generally will be paid in cash (or its equivalent) in a single lump sum. The Compensation Committee reserves the right to settle an actual award with a grant of an equity award with such terms and conditions, including any vesting requirements, as determined by the Compensation Committee. Unless otherwise determined by our Compensation Committee, to earn an actual award, a participant must be employed by us (or an affiliate of us, as applicable) on the date the bonus is paid. Payment of bonuses occurs as soon as practicable after the end of the applicable performance period, but no later than the dates set forth in the Bonus Plan.
All awards under the Bonus Plan will be subject to reduction, cancellation, forfeiture, or recoupment in accordance with our Compensation Recovery Policy (as described below). Our Compensation Committee may also impose such other clawback, recovery or recoupment provisions with respect to an award under the Bonus Plan as it may determine is necessary or appropriate.
If we are required to prepare an accounting restatement due to our material noncompliance, as a result of misconduct, with any financial reporting requirement under the securities laws, then any participant who knowingly or through gross negligence engaged in, or failed to prevent, the misconduct, will reimburse us for the amount of any payment with respect to an award earned or accrued under the Bonus Plan during the twelve month period following the first to occur of the public issuance or filing with the SEC, of the financial document embodying such financial reporting requirement.
Our Board or its Compensation Committee will have the authority to amend or terminate the Bonus Plan provided such action does not alter or impair the existing rights of any participant with respect to any earned bonus without the participant’s consent. The Bonus Plan will remain in effect until terminated in accordance with the terms of the Bonus Plan.
For the fiscal year ended December 31, 2023, annual bonuses were determined based on target bonuses set for each employee, including our NEOs. The extent to which a target bonus was achieved was determined based on our performance against a number of corporate-level goals, including the achievement of milestones in the lead program, pipeline development, investor relations, and operations (the “2023 Performance Goals”). In January 2023, the Compensation Committee reviewed the actual achievements against the 2022 Performance Goals and determined that the payout for annual bonuses would be capped at 90%. The table below provides additional details about the 2022 Performance Goals and the Compensation Committee’s assessment of our actual performance against the 2022 Performance Goals.
| 2023 Performance Goals | Weighting | Performance | Weighted Performance Achievement | |||||||||
| Lead Program | 45.0 | % | 94.3 | % | 42.4 | % | ||||||
| Pipeline Development | 10.0 | % | 100.0 | % | 10.0 | % | ||||||
| Investor Relations | 35.00 | % | 58.9 | % | 20.6 | % | ||||||
| Breakthrough Designation | 5.0 | % | 100.0 | % | 5.0 | % | ||||||
| Operations | 5.0 | % | 40.0 | % | 2.0 | % | ||||||
| Total | 100.0 | % | — | 80.0 | % | |||||||
Mr. Bagai has an established annual bonus target of 50%, Dr. Agah has an established annual bonus target of 40% and, Ms. Nelms has an established annual bonus target of 35%. Using the above achievement level and making additional adjustments based on the start date, the Compensation Committee determined to conserve the Company’s cash position and that the bonus awards for 2023 was to grant fully vested non-qualified options with a 10-year term issued to the NEOs as follows:
| Executive | Salary ($) | Target Bonus | 2023 Earned Bonus In Fully Vested Options | |||||||||
| Shaun Bagai | 520,000 | 55 | % | 69,376 | ||||||||
| Ramtin Agah | 303,450 | 40 | % | 29,444 | ||||||||
| Angela Gill Nelms | 412,000 | 35 | % | 34,980 | ||||||||
| 114 |
Compensation Recovery Policy
On September 7, 2023, our Board of Directors adopted a policy (commonly known as a “clawback” policy) which provides for the recovery of erroneously awarded incentive compensation to certain of our officers in the event that we are required to prepare an accounting restatement due to material noncompliance by us with any financial reporting requirements under the federal securities laws. This policy is designed to comply with Section 10D of the Securities Exchange Act of 1934, as amended, related rules and the listing standards of Nasdaq Stock Market or any other securities exchange on which our shares are listed in the future. The policy is administered by our Compensation Committee. Any determinations made by the Compensation Committee shall be final and binding on all affected individuals.
The individuals covered by this policy (the “Executive Officers”) are any current or former employee who is or was identified as our president, principal financial officer, principal accounting officer (or if there is no such accounting officer, the controller), any vice-president in charge of a principal business unit, division, or function (such as sales, administration, or finance), any other officer who performs a policy-making function, or any other person (including any executive officer of our subsidiaries or affiliates) who performs similar policy-making functions for us.
The policy covers our recoupment of “Clawback Eligible Incentive-Based Compensation” (as defined in the policy) received by a person after beginning service as an Executive Officer and who served as an Executive Officers at any time during the performance period for that Clawback Eligible Incentive-Based Compensation. In the event we are required to prepare an accounting restatement, the policy requires us to recover, reasonably promptly, any Clawback Eligible Incentive-Based Compensation (as determined by our Compensation Committee) received by any Executive Officer during the three completed fiscal years immediately preceding the date on which we are required to prepare such accounting restatement.
The foregoing description of our Compensation Recovery Policy does not purport to be complete and is qualified in its entirety by the terms and conditions of such policy, a copy of which is filed as an exhibit to this Report and is incorporated herein by reference.
Equity Compensation Plan Information
All of our equity compensation plans have been approved by our stockholders. The following table provides information as of December 31, 2023, with respect to the shares of our common stock that may be issued under our existing equity compensation plans.
| Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a))(1) | ||||||||||
| Plan Category | (a) | (b) | (c) | |||||||||
| 2013 Equity Incentive Plan | 474,780 | $ 0. 66 | ||||||||||
| 2021 Omnibus Equity Incentive Plan | 1,385,345 | $ 3. 09 | 1,387,691 | |||||||||
| Total | 1,860,125 | $ 2. 40 | 1,387,691 | |||||||||
| (1) | The 2021 Plan provides an annual increase on the first day of each calendar year beginning with January 1, 2022 and ending with the last January 1 during the initial ten-year term of the 2021 Plan, equal to the lesser of (a) three percent (3%) of the shares outstanding on the final day of the immediately preceding calendar year; and (b) such lesser number of shares as determined by the Board. |
Director Compensation
We have adopted a director compensation program with respect to compensation payable to our non-employee directors for their service as directors. From time to time, we have granted equity awards to attract them to join our Board and for their continued service on our Board. We also have reimbursed our directors for expenses associated with attending meetings of our Board and its committees.
In setting director compensation, including any modifications to the director compensation program, the Compensation Committee considers several factors including our size and stage of development and market data of our peer group. The Compensation Committee also considers recommendations from its independent compensation consultant in making these determinations.
The following table provides information regarding compensation of our non-employee directors, other than Mr. Bagai and Dr. Agah who are also a NEOs, for service as directors for the fiscal year ended December 31, 2023. Mr. Bagai and Dr. Agah does not receive any additional compensation as a director. For information on Dr. Agah’s compensation, see – “Summary Compensation Table.”
| Name | Fees Earned or Paid in Cash ($) | Options Awards(1)(2) ($) | Total Compensation ($) | |||||||||
| David Diamond | 61,000 | 30,995 | (3) | 91,995 | ||||||||
| Kirsten Angela Macfarlane | 51,000 | 30,995 | (4) | 81,995 | ||||||||
| Laurence J. Marton, M.D. | 46,000 | 30,995 | (4) | 76,995 | ||||||||
| Una S. Ryan, O.B.E., Ph.D., D.Sc. | 51,000 | 30,995 | (4) | 81,995 | ||||||||
| Robert Spiegel, M.D., FACP | 35,540 | 138,181 | (5) | 173,721 | ||||||||
| (1) | The number of unexercised stock options held by the directors named in the above table as of December 31, 2023, was as follows: Mr. Diamond (71,496), Ms. Macfarlane (77,018), Dr. Marton (97,498), Dr. Ryan (61,018) and Dr. Spiegel (13,744). |
| (2) | The amounts in this column represent the aggregate grant date fair value of the stock option awards computed in accordance with FASB guidance on stock-based compensation. The relevant assumptions made in the valuations for the 2023 and 2022 stock option awards may be found in Note 7 of the Notes to the accompanying audited financial statements. The grant date fair value of stock options is determined based on the number of options awarded and the fair value of the stock option on the grant date, which is the Black Scholes value of closing sales price per share of our common stock. |
| (3) | Represents a stock option granted to the non-employee director on October 1, 2023, to purchase 25,099 shares of our common stock, which vests in twelve equal monthly installments beginning on November 1, 2023. Mr. Diamond passed away in late December 2023. |
| (4) | Represents a stock option granted to the non-employee director on October 1, 2023, to purchase 25,099 shares of our common stock, which vests in twelve equal monthly installments beginning on November 1, 2023. |
| (5) | Represents (i) an initial stock option granted to the non-employee director on April 25, 2023, to purchase 43,026 shares of our common stock, which vests in 36 equal monthly installments beginning on May 25, 2023, and (ii) and additional 2023 grant of an option to purchase 25,099 shares of our common stock, which vest in twelve equal monthly installments beginning on November 1, 2023. |
| 115 |
Outside Director Compensation Policy
Under our Outside Director Compensation Policy, each non-employee director receives a cash and equity compensation for his or her services as a member of our Board, as described below. We also will continue to reimburse our non-employee directors for reasonable, customary, and documented travel expenses to meetings of our Board or its committees.
The Outside Director Compensation Policy includes a maximum annual limit of $250,000 of cash compensation and equity awards that may be paid, issued, or granted to a non-employee director in any fiscal year (increased to $300,000 in the fiscal year in which the non-employee director joins the Board). For purposes of these limitations, the value of an equity award is based on its grant date fair value. Any cash compensation paid or equity awards granted to a person for his or her services as an employee, or for his or her services as a consultant (other than as a non-employee director), will not count for purposes of the limitation. The maximum limit does not reflect the intended size of any potential compensation or equity awards to our non-employee directors.
On September 29, 2022, the Outside Director Compensation Policy was amended to increase the value of the automatic annual option awards to $70,000 and to reflect various clarifications, including but not limited to the mechanism by which the value of stock options is determined and the maximum number of options allowed to be issued for the Initial Award (defined below) and Annual Award (defined below), respectively.
Cash Compensation
Non-employee directors are paid an annual cash retainer of $36,000. In addition, each non-employee director who serves as the chair or a member of a committee of the Board will be eligible to earn additional annual cash fees as follows:
| ● | $15,000 per year for service as Chair of the Audit Committee; | |
| ● | $10,000 per year for service as Chair of the Compensation Committee; | |
| ● | $10,000 per year for service as Chair of the Nominating and Corporate Governance Committee; and | |
| ● | $5,000 per year for service as a member of each of the Audit, Compensation, and Nominating and Corporate Governance committees. | |
| ● | $5,000 per year for service as Lead Independent Director |
Equity Compensation
Initial Options
Each individual who first becomes a non-employee director will be granted options at a grant date fair value of approximately $120,000 in the aggregate (an “Initial Award”), provided that no Initial Award granted on or after September 29, 2022 but on or prior to September 30, 2023 will cover more than 43,026 shares of common stock, subject to certain adjustments, on the date on which such individual first becomes a non-employee director, whether through election by our stockholders or appointment by the Board to fill a vacancy. Each Initial Award will vest and become exercisable over three years, with 1/36th of the Initial Award vesting each month on the same day of the month as the commencement of the applicable individual’s service as non-employee director.
Annual Options
On October 1 of each year, commencing October 1, 2022, each non-employee director will be automatically granted options at a grant date fair value of $70,000 in the aggregate (an “Annual Award”), provided that no Annual Award granted on or after September 29, 2022 but on or prior to September 30, 2023 will cover more than 25,099 shares of common stock, subject to certain adjustments. Each Annual Award will vest and become exercisable over 12 months, with 1/12th of the Annual Award vesting monthly after October 1 on the first day of each subsequent month. On October 1, 2022, each non-employee director was granted options at a fair market value of $50,000. These options vest and become exercisable over 12 months, with 1/12th of the Annual Award vesting monthly after October 1 on the first day of each subsequent month.
Change in Control
In the event of our “change in control” (as defined in the Outside Director Compensation Policy), each non-employee director will fully vest in his or her outstanding company equity awards provided that the non-employee director continues to be a non-employee director through the date of our change in control.
Compensation Committee Interlocks and Inside Participation
None of the members of our Compensation Committee is or has been an officer or employee of our company. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the Board of Directors or Compensation Committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire Board of Directors) of any entity that has one or more executive officers serving on our Board of Directors or Compensation Committee.
| 116 |
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table shows information regarding the beneficial ownership of our common stock as of the date of this Report for the following:
| ● | Each stockholder known by us to beneficially own more than 5% of our common stock; | |
| ● | Each of our named executive officers; | |
| ● | Each of our directors; and | |
| ● | All of our executive officers and directors as a group. |
The table is based on information supplied to us by directors, executive officers and principal stockholders and filings under the Exchange Act. We have based our calculation of the percentage of beneficial ownership on 16,826,994 shares of our common stock issued and outstanding as of March 7, 2024, unless otherwise noted. The beneficial ownership reported in the following table is determined in accordance with the applicable rules of the SEC and does not necessarily indicate beneficial ownership for any other purpose. For purposes of the following tables, an entity or individual is considered the beneficial owner of shares of common stock if he or she has the right to acquire within 60 days of March 7, 2024, such common stock and directly or indirectly has or shares voting power or investment power, as defined in the rules of the SEC, with respect to such shares.
Except as indicated in the footnotes below, the address of the persons or groups named below is c/o RenovoRx, Inc., 4546 El Camino Real, Suite B1 Los Altos, California 94022.
| Name of Beneficial Owner | Shares | Percentage | ||||||
| 5% stockholders: | ||||||||
| Estate of Kamran Najmabadi(1) | 1,039,500 | 6.2 | % | |||||
| Bank of the West(2) | 975,000 | 5.8 | % | |||||
| Named Executive Officers and Directors: | ||||||||
| Shaun R. Bagai.(3) | 758,177 | 4.5 | % | |||||
| Ramtin Agah, M.D.(4) | 320,676 | 1.9 | % | |||||
| Ronald B. Kocak (5) | 38,181 | * | ||||||
| Leesa Gentry (6) | 47,967 | * | ||||||
| Kirsten Angela Macfarlane(7) | 169,442 | 1.0 | % | |||||
| Laurence J. Marton, M.D.(8). | 149,786 | * | ||||||
| Una S. Ryan, O.B.E., Ph.D., D.Sc.(9) | 71,496 | * | ||||||
| Robert Spiegel, M.D., FACP(10) | 147,359 | * | ||||||
| All current executive officers and directors as a group (8 persons)(11) | 1,666,654 | 9.9 | % | |||||
| * | Represents less than 1% of the outstanding shares of our common stock. |
| (1) | Consists of (i) 741,250 shares held by The Najmabadi Family Trust dated April 22, 2021; (ii) 121,875 shares of common stock held by The Navid Najmabadi Irrevocable Trust dated April 22, 2021; (iii) 121,875 shares of common stock held by The Leili Najmabadi Irrevocable Trust dated April 22, 2021 and (iv) 54,500 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024. |
| (2) | Based solely on the Schedule 13G filed with the SEC on April 14, 2022. Consists of 975,000 shares of common stock over which Bank of the West, Trustee of the Mars16 Nevada Non-Grantor Trust Under Agreement Dated January 19, 2016 has sole voting power. The address of Bank of the West is 180 Montgomery Street, San Francisco, California 94104. |
| (3) | Consists of (i) 292,450 shares of common stock held by Mr. Bagai and (ii) 424,654 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024,and (iii) 40,983 shares of common stock underlying warrants exercisable within 60 days of March 7, 2024. |
| (4) | Consists of (i) 47,460 shares of common stock held by Dr. Agah; (ii) 230,938 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024 and (iii) 42,278 shares of common stock underlying warrants exercisable within 60 days of March 7, 2024. |
| (5) | Consists of 38,181 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024. |
| (6) | Consists of 47,967 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024. |
| (7) | Consists of (i) 40,983 shares of common stock held by Ms. Macfarlane; (ii) 87,476 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024 and (iii) 40,983 shares of common stock underlying warrants exercisable within 60 days of March 7, 2024. |
| (8) | Consists of (i) 33,634 shares of common stock held by Mr. Marton; (ii) 107,956 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024 and (iii) 8,196 shares of common stock underlying warrants exercisable within 60 days of March 7, 2024. |
| (9) | Consists of 71,476 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024. |
| (10) | Consists of (i) 40,983 shares of common stock held by Mr. Speigel; (ii) 28,983 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024 and (iii) 40,983 shares of common stock underlying warrants exercisable within 60 days of March 7, 2024. |
| (11) | Consists of (i) 455,600 shares of common stock held by our current executive officers and directors; (ii) 1,037,631 shares of common stock underlying stock options exercisable within 60 days of March 7, 2024 and (iii) 173,423 shares of common stock underlying warrants exercisable within 60 days of March 7, 2024. |
| 117 |
Item 13. CERTAIN RELATIONSHIPS AND RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The following includes a summary of transactions since January 1, 2023 to which we have been a party in which the amount involved exceeded or will exceed the lesser of $120,000 or 1% of the average of our total assets as of December 31, 2023 and 2022, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other arrangements, which are described under the sections titled “Executive Compensation,” “Director Compensation,” and “Certain Relationships and Related Party Transactions.”
Policies and Procedures for Related Person Transactions
Our Board has adopted a written related person transaction policy, setting forth the policies and procedures for the review and approval or ratification of related person transactions. This policy covers, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, in which we were or are to be a participant, where the amount involved exceeds the lesser of $120,000 or 1% of the average of our total assets at year-end for the last two completed fiscal years and a related person had, has or will have a direct or indirect material interest, including without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our Audit Committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
Employee, Officer and Director Hedging
We believe it is improper and inappropriate for any person associated with us to engage in short-term or speculative transactions involving our securities. Our Insider Trading Policy prohibits our directors, officers and employees from engaging in any such transactions (including prepaid variable forwards, equity swaps, collars and exchange funds).
Other Transactions
We have granted stock options to our NEOs and our directors. See the sections titled “Director Compensation” and “Executive Compensation,” for a description of these stock options. In the ordinary course of business, we enter into offer letters and employment agreements with our executive officers. We have also entered into indemnification agreements with each of our directors and officers. The indemnification agreements and our Certificate of Incorporation and Bylaws require us to indemnify our directors and officers to the fullest extent permitted by Delaware law.
| 118 |
Director Independence
Our common stock is listed on The Nasdaq Capital Market. As a company listed on The Nasdaq Capital Market, we are required under the Marketplace Rules of The Nasdaq Stock Market (the “Nasdaq Marketplace Rules”) to maintain a board comprised of a majority of independent directors as determined affirmatively by our Board. Under the Nasdaq Marketplace Rules, a director will only qualify as an “independent director” if, in the opinion of that listed company’s board of directors, the director does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In addition, the Nasdaq Marketplace Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees be independent.
Audit committee members must also satisfy the additional independence criteria set forth in Rule 10A-3 under the Exchange Act and Nasdaq Marketplace Rules applicable to audit committee members. Compensation committee members must also satisfy the additional independence criteria set forth in Rule 10C-1 under the Exchange Act and Nasdaq Marketplace Rules applicable to compensation committee members.
Our Board of Directors has undertaken a review of the independence of each of our directors. Based on information provided by each director concerning his or her background, employment, and affiliations, our Board of Directors has determined that Ms. Macfarlane, Dr. Marton, M.D., Dr. Ryan, O.B.E., Ph.D., D.Sc., and Dr. Spiegel, M.D., FACP representing five of our six directors, do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is an “independent director” as defined under the Nasdaq Marketplace Rules. Shaun R. Bagai and Ramtin Agah, M.D., are not considered independent directors because of their status as Chief Executive Officer and Chief Medical Officer, respectively.
In making these determinations, our Board of Directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our Board of Directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director, and the transactions involving them described in the section titled “Certain Relationships and Related Party Transactions.” There are no family relationships among any of our directors, director nominees or executive officers.
| 119 |
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Audit and Non-Audit Fees
Baker Tilly US, LLP served as our independent registered public accounting firm for the fiscal year ended December 31, 2023. The following table sets forth the fees accrued or paid to Baker Tilly US, LLP, in connection with our December 31, 2023 and 2022 audit.
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Audit Fees | $ | 215,000 | $ | 226,275 | ||||
| Audit Related Fees | 71,012 | (1) | 21,000 | |||||
| Tax Fees | - | - | ||||||
| All Other Fees | - | - | ||||||
| Total | 286,012 | 247,275 | ||||||
(1) Represents professional fees for fiscal year 2023 consent of registration statements.
For the fiscal year ended December 31, 2022, we paid Frank Rimerman + Co. LLP $30,000 in audit related fees.
Pre-Approval of Audit and Non-Audit Services
In accordance with its Charter, the Audit Committee pre-approves all audit and permitted non-audit and tax services provided by our independent registered public accounting firm.
Prior to the annual engagement of our independent registered public accounting firm, the Audit Committee pre-approves all services to be provided. During the year, circumstances may arise when it may become necessary to engage the independent registered public accounting firm for additional services. In such circumstances, our management seeks approval of the non-audit services that it recommends the Audit Committee engage the independent registered public accounting firm to provide for the fiscal year. A budget, estimating the specific non-audit service spending for the fiscal year, is provided to the Audit Committee along with the request. The Audit Committee will be regularly informed of the non-audit services actually provided by the independent auditor pursuant to this pre-approval process. All fees accrued or paid to Baker Tilly US, LLP in connection with our December 31, 2023 and 2022 audit.
| 120 |
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this Annual Report on Form 10-K
1. Financial Statements
| Page | |
| Financial Statements for the Years Ended December 31, 2023 and 2022 | |
| Report of Independent Registered Public Accounting Firm Baker Tilly US, LLP (PCAOB Firm ID |
F-1 |
| Balance Sheets | F-2 |
| Statements of Operations and Comprehensive Loss | F-3 |
| Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit) | F-4 |
| Statements of Cash Flows | F-5 |
| Notes to Financial Statements | F-6 |
| 121 |
2. Financial Statement Schedules
All financial statement schedules have been omitted because the required information is not applicable or is not present in amounts sufficient to require submission of the schedules, or because the information required is included in the financial statements and accompanying notes included in this Report.
3. Exhibits
See “Exhibit Index” immediately preceding the signature page of this Report, which is incorporated herein by reference.
ITEM 16. FORM 10-K SUMMARY
None.
RENOVORX, INC.
EXHIBIT INDEX
| Incorporated by Reference | ||||||||||
| Exhibit No. | Exhibit Description | Form | File No. | Exhibit | Filing Date | |||||
| 3.1 | Sixth Amended and Restated Certificate of Incorporation of RenovoRx, Inc. | 8-K | 001-40738 | 3.1 | August 31, 2021 | |||||
| 3.2 | Amended and Restated Bylaws of RenovoRx, Inc. | 8-K | 001-40738 | 3.1 | September 11, 2023 | |||||
| 4.1 | Form of Private Common Stock Warrant (related to the 2020 Convertible Notes and 2021 Convertible Notes) | 10-Q | 001-40738 | 4.1 | November 15, 2021 | |||||
| 4.2 | Form of Underwriter’s Warrant | S-1 | 333-258071 | 4.1 | August 25, 2021 | |||||
| 4.3 | Form of Warrant Agent Agreement (including the terms of the Warrants) | S-1 | 333-258071 | 4.2 | August 25, 2021 | |||||
| 4.4 | Specimen Stock Certificate evidencing the Shares of Common Stock | S-1 | 333-258071 | 4.4 | August 25, 2021 | |||||
| 4.5 | Form of Warrant Certificate | S-1 | 333-258071 | 4.5 | August 25, 2021 | |||||
| 4.6 | Form of Pre-Funded Common Stock Purchase Warrant | 8-K | 001-40738 | 4.1 | April 3, 2023 | |||||
| 4.7 | Form of Common Stock Purchase Warrant | 8-K | 001-40738 | 4.2 | April 3, 2023 | |||||
| 122 |
| 123 |
| 10.16† | Form of Placement Agency Agreement by and between RenovoRx, Inc. and Roth Capital Partners, LLC, dated March 30, 2023 | 8-K | 001-40738 | 10.1 | April 3, 2023 | |||||
| 10.17† | Form of Securities Purchase Agreement | 8-K | 001-40738 | 10.2 | April 3, 2023 | |||||
| 10.18 | Subscription Agreement, by and between RenovoRx, Inc. and the investors thereto | 8-K | 001-40738 | 10.1 | January 29, 2024 | |||||
| 10.19 | Offering Extension, dated January 12, 2024 | 8-K | 001-40738 | 10.2 | January 29, 2024 | |||||
| 10.20 | Placement Agent Agreement, by and between RenovoRx, Inc. and Paulson Investment Company, LLC, dated November 14, 2023 | 8-K | 001-40738 | 10.4 | January 29, 2024 | |||||
| 10.21 | Amended and Restated Offer Letter, by and between RenovoRx, Inc. and Ronald B. Kocak | 8-K | 001-40738 | 10.1 | February 9, 2024 | |||||
| 10.22 | Amended and Restated Offer Letter, by and between RenovoRx, Inc. and Leesa Gentry | 8-K | 001-40738 | 10.1 | March 14, 2024 | |||||
| 19.1 | Insider Trading Policy | Filed herewith | ||||||||
| 23.1 | Consent of Baker Tilly US, LLP | Filed herewith | ||||||||
| 31.1 | Certification of Principal Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a). | Filed herewith | ||||||||
| 31.2 | Certification of Principal Accounting Officer Pursuant to Rule 13a-14(a)/15d-14(a). | Filed herewith | ||||||||
| 32.1* | Certification of Principal Executive Officer Pursuant to 18 U.S.C. Section 1350. | Furnished herewith | ||||||||
| 32.2* | Certification of Principal Accounting Officer Pursuant to 18 U.S.C. Section 1350. | Furnished herewith | ||||||||
| 97.1 | Policy Related to Recovery of Erroneously Awarded Compensation, adopted September 7, 2023 | Filed herewith | ||||||||
| 101.INS | Inline XBRL Taxonomy Extension Instance Document (the instance document does not appear on the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document). | Filed herewith | ||||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | Filed herewith | ||||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | Filed herewith | ||||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | Filed herewith | ||||||||
| 101.LAB | Inline XBRL Taxonomy Extension Labels Linkbase Document | Filed herewith | ||||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | Filed herewith | ||||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in the Interactive Data Files submitted as Exhibit 101). | Filed herewith |
† Indicates management contract or compensatory plan or arrangement
+ Confidential portions of this Exhibit were redacted pursuant to Item 601(b)(2) of Regulation S-K and RenovoRx, Inc. agrees to furnish supplementally to the Securities and Exchange Commission a copy of any redacted information or omitted schedule and/or exhibit upon request.
* The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report on Form 10-K are deemed furnished and not filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of the Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report, irrespective of any general incorporation language contained in such filing.
| 124 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| RENOVORX, INC. | |
| Date: April 1, 2024 | /s/ Shaun R. Bagai |
| Shaun R. Bagai | |
| Chief Executive Officer | |
| Date: April 1, 2024 | /s/ Ronald B. Kocak |
| Ronald B. Kocak | |
| VP Controller and Principal Accounting Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Shaun R. Bagai | Chief Executive Officer, Director | April 1, 2024 | ||
| Shaun R. Bagai | (Principal Executive Officer) | |||
| /s/ Ronald B. Kocak | VP, Controller and Principal Accounting Officer | April 1, 2024 | ||
| Ronald B. Kocak | (Principal Accounting Officer) | |||
| /s/ Ramtin Agah | Chairman of the Board of Directors | April 1, 2024 | ||
| Ramtin Agah, M.D. | ||||
| /s/ Laurence J. Marton | Director | April 1, 2024 | ||
| Laurence J. Marton, M.D. | ||||
| /s/ Una S. Ryan | Director | April 1, 2024 | ||
| Una S. Ryan, O.B.E., Ph.D., D.Sc. | ||||
| /s/ Kirsten Angela Macfarlane | Director | April 1, 2024 | ||
| Kirsten Angela Macfarlane | ||||
| /s/ Robert J. Spiegel | Director | April 1, 2024 | ||
| Robert J. Spiegel, M.D., FACP |
| 125 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of RenovoRx, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of RenovoRx, Inc. (the “Company”) as of December 31, 2023 and 2022, the related statements of operations and comprehensive loss, convertible preferred stock and stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes to the financial statements (collectively the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt About the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred significant losses and negative cash flows from operations since its inception. The Company incurred a net loss of $10.2 million during the year ended December 31, 2023, and reported an accumulated deficit of $41.4 million as of December 31, 2023. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/
We have served as the Company’s auditor since 2021.
April 1, 2024
| F-1 |
RenovoRx, Inc.
Balance Sheets
(in thousands, except share and per share amounts)
| As of December 31, | ||||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Short-term marketable securities | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Deferred offering costs | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities, Convertible Preferred Stock and Stockholders’ Equity (Deficit) | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Total current liabilities | ||||||||
| Common stock warrant liability | ||||||||
| Total liabilities | $ | $ | ||||||
| Commitments and contingencies (Note 6) | ||||||||
| Convertible preferred stock and stockholders’ equity (deficit): | ||||||||
| Convertible preferred stock, $ par value; shares authorized as of December 31, 2023 and 2022, respectively; shares issued and outstanding at December 31, 2023 and 2022, respectively | ||||||||
| Common stock, $ par value, shares authorized at December 31, 2023, and 2022, respectively; and shares issued and outstanding as of December 31, 2023, and 2022, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated other comprehensive income | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total convertible preferred stock and stockholders’ (deficit) equity | ( | ) | ||||||
| Total liabilities, convertible preferred stock and stockholders’ equity (deficit) | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements.
| F-2 |
RenovoRx, Inc.
Statements of Operations and Comprehensive Loss
(in thousands, except share and per share amounts)
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Change in fair value of warrant liability | ||||||||
| Interest income (expense), net | ||||||||
| Other income, net | ||||||||
| Financing costs allocated to warrant | ( | ) | ||||||
| Total other income (expenses), net | ||||||||
| Net loss | ( | ) | ( | ) | ||||
| Other comprehensive income: | ||||||||
| Unrealized gain on marketable securities | ||||||||
| Comprehensive loss | $ | ( | ) | $ | ( | ) | ||
| Net loss per share, basic and diluted | $ | ) | $ | ) | ||||
| Weighted-average shares of common stock outstanding, basic and diluted | ||||||||
The accompanying notes are an integral part of these financial statements.
| F-3 |
RenovoRx, Inc.
Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands, except share amounts)
Convertible Preferred Stock |
Common Stock | Additional Paid-In | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ Equity | |||||||||||||||||||||||||||
| Shares | Amount |
Shares | Amount |
Capital |
Income |
Deficit | (Deficit) | |||||||||||||||||||||||||
| Balance—December 31, 2021 | $ | $ | | $ | $ | $ | ( | ) | $ | | ||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options | - | |||||||||||||||||||||||||||||||
| Issuance of restricted stock awards | - | |||||||||||||||||||||||||||||||
| Stock-based compensation expense | - | - | ||||||||||||||||||||||||||||||
| Other comprehensive income | - | - | ||||||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Balance—December 31, 2022 | ( | ) | ||||||||||||||||||||||||||||||
| Issuance of common stock upon exercise of stock options | - | |||||||||||||||||||||||||||||||
| Issuance of restricted stock awards | - | |||||||||||||||||||||||||||||||
| Issuance of common stock upon the registered direct offering | - | |||||||||||||||||||||||||||||||
| Issuance and exercise of common stock warrants upon the registered direct offering | - | |||||||||||||||||||||||||||||||
| Stock-based compensation expense | - | - | ||||||||||||||||||||||||||||||
| Other comprehensive income | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Balance—December 31, 2023 | $ | $ | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
| F-4 |
RenovoRx, Inc.
Statements of Cash Flows
(in thousands)
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock-based compensation expense | ||||||||
| Amortization on leasehold improvements | ||||||||
| Change in fair value of common stock warrants classified as a liability | ( | ) | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Other assets | ( | ) | ||||||
| Accounts payables | ||||||||
| Accrued expenses | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of marketable securities | ( | ) | ||||||
| Proceeds from maturities of marketable securities | ||||||||
| Proceeds from the sale of investments | ||||||||
| Net cash provided by (used in) investing activities | ( | ) | ||||||
| Cash flows from financing activities: | ||||||||
| Proceeds from common stock and pre-funded common stock warrants | ||||||||
| Proceeds from exercise of stock options | ||||||||
| Net cash provided by financing activities | ||||||||
| Net decrease in cash and cash equivalents | ( | ) | ( | ) | ||||
| Cash and cash equivalents: | ||||||||
| Beginning of year | ||||||||
| End of year | $ | $ | ||||||
| Supplemental of non-cash financing activities: | ||||||||
| Fair value of common warrant classified as a liability | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements.
| F-5 |
RenovoRx, Inc.
Notes to Financial Statements
1. Business and Principal Activities
Description of Business
RenovoRx, Inc. (the “Company”) was incorporated in the state of Delaware in December 2012 and operates from its headquarters in Los Altos, California. The Company is a clinical-stage biopharmaceutical company focused on developing therapies for the local treatment of solid tumors and conducting a Phase III pancreatic cancer clinical trial for its lead product candidate RenovoGem™. The Company’s therapy platform, RenovoRx Trans-Arterial Micro-Perfusion, or TAMP® utilizes approved chemotherapeutics with validated mechanisms of action and well-established safety and side effect profiles, with the goal of increasing their efficacy, improving their safety, and widening their therapeutic window.
Liquidity, Capital Resources and Substantial Doubt About the Company’s Ability to Continue as a Going Concern
From
the Company’s inception through December 31, 2023, it has raised an aggregate of $
The
Company has incurred significant losses and negative cash flows from operations since its inception. For the year ended December 31,
2023, the Company reported a net loss of $
The
Company has filed an omnibus shelf registration statement on Form S-3 that provides for the aggregate offerings of up to $
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern and has reviewed the relevant conditions and events surrounding its ability to continue as a going concern including among others: historical losses, projected future results, negative cash flows from operations, including cash requirements for the upcoming year, funding capacity, net working capital, total stockholders’ equity and future access to capital. Based upon this review and the Company’s current financial condition, the Company has concluded that substantial doubt exists as to the Company’s ability to continue as a going concern.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and applicable rules and regulations of the SEC for annual reporting. Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and as amended by Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
| F-6 |
Risks and Uncertainties
The Company is subject to a number of risks associated with companies at a similar stage, including the risk associated with the development of products that must receive regulatory approval before market launch, dependence on key individuals, competition from larger and established companies, volatility of the industry, ability to obtain adequate financing to support growth, the ability to attract and retain additional qualified personnel to manage the anticipated growth of the Company and general economic conditions. The Company is subject to a number of risks similar to other early-stage biopharmaceutical companies, including, but not limited to, the need to obtain adequate additional funding, possible failure of current or future preclinical studies or clinical trials, its reliance on third parties to conduct its clinical trials, the need to obtain regulatory and marketing approvals for its product candidates, competitors developing new technological innovations, the need to successfully commercialize and gain market acceptance of the Company’s product candidates, protection of its proprietary technology, and the need to secure and maintain adequate manufacturing arrangements with third parties.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, income and expenses as well as the disclosure of contingent assets and liabilities, at the date of the financial statements during the reporting periods. In preparing these financial statements, management has made its best estimates and judgments of certain amounts included in the financial statements. Significant estimates and assumptions made in the accompanying financial statements include, but are not limited to, accruals of certain liabilities, including clinical trial accruals and other contingences, the valuation of financial instruments, the fair value of the Company’s common stock and the fair value of options granted under the Company’s equity incentive plan. On an ongoing basis, the Company evaluates its estimates, including those related to the fair values of assets, stock-based compensation, clinical trial accruals and other contingencies. Management bases its estimates on historical experience or on various other assumptions that it believes to be reasonable under the circumstances. Actual results could differ materially from these estimates.
Concentration of Credit Risk and Significant Suppliers
Financial
instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents.
The Company maintains bank deposits in federally insured financial institutions and these deposits may exceed federally insured limits
of $
The Company relied, and expects to rely, on a small number of third-party manufacturers to manufacture and supply its RenovoCath devices and its product candidates for clinical trials. These activities could be adversely affected by a significant interruption in supply of these items. If the Company does not successfully commercialize or partner any of its product candidates, it will be unable to generate product revenue or achieve profitability.
Operating Segment
The
Company operates and manages its business as
| F-7 |
Basic net loss per common share is calculated by dividing net loss by the weighted-average number of shares of common stock outstanding during the period, without consideration of common stock equivalents. Diluted net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock and common stock equivalents of potentially dilutive securities outstanding for the period determined using the treasury stock and if-converted methods. Potentially dilutive common stock equivalents are comprised of convertible preferred stock, convertible notes, and warrants including options and restricted stock awards outstanding under the Company’s Equity Incentive Plan. For the years ended December 31, 2023 and 2022, there was no difference in the number of shares used to calculate basic and diluted shares outstanding as the inclusion of the potentially dilutive securities would be anti-dilutive.
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability, or an exit price, in the principal or most advantageous market for that asset or liability in an orderly transaction between market participants on the measurement date. Fair value measurement establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs, where available, and minimize the use of unobservable inputs when measuring fair value.
The Company determined the fair value of financial assets and liabilities using the fair value hierarchy that describes three levels of inputs that may be used to measure fair value, as follows:
Level 1 – Valuations based on quoted prices for identical assets and liabilities in active markets.
Level 2 – Valuations based on observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data.
Level 3 – Valuations based on unobservable inputs reflecting the Company’s assumptions, consistent with reasonably available assumptions made by other market participants. These valuations require significant judgment.
The estimated fair value of financial instruments disclosed in the financial statements has been determined by using available market information and appropriate valuation methodologies. In certain cases where there is limited activity or less transparency around inputs to valuation, securities are classified as Level 3.
The carrying amount of current assets, accounts payable and accrued expenses are generally considered to be representative of their respective fair values because of their short-term nature.
Cash, Cash Equivalents and Investments
The Company considers all highly liquid investments with original maturities of three months or less when purchased to be cash equivalents. Available-for-sale securities, comprised of obligations of the U.S. government and its agencies, are carried at fair value, with unrealized gains and losses recorded within other comprehensive income (loss) as a separate component of stockholders’ equity. Realized gains and losses and declines in value judged to be other than temporary, if any, on available-for-sale securities are included in earnings. Purchases and sales of investment securities are recognized on a trade date basis. The cost of investment securities sold is determined by the specific identification method.
Leasehold Improvements, Net
Leasehold improvements are presented at cost, net of accumulated amortization. Amortization expense is recorded using the straight-line method over the shorter of the remaining lease term or the estimated useful life.
| F-8 |
Research and Development Costs
Research
and development expenses are charged to expense as incurred. Research and development expenses includes personnel costs including salaries,
benefits and stock-based compensation. In addition, it includes expenses for consultants that support clinical trial studies, materials
costs, external clinical drug product manufacturing costs, outside services costs, regulatory activities including filing fees, fees
for maintaining licenses and other amounts due to third-party agreements, laboratory materials, clinical trial, as noted above, and supplies
to support our research activities, including allocated facility and general and administrative indirect overhead related costs. The
Company also receives payments from clinical trial sites for RenovoCath delivery devices used in the Phase III clinical trial. Payments
received from clinical sites cover the direct costs of manufacturing the RenovoCath delivery devices and offset research and development
expenses were $
Clinical Trial Expenses
The Company makes payments in connection with its ongoing Phase III clinical trial under contracts with clinical trial sites and contract research organizations that support conducting and managing clinical trials. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Generally, these agreements set forth the scope of work to be performed at a fixed fee, unit price or on a time and materials basis. A portion of the obligation to make payments under these contracts depends on factors such as the successful enrollment or treatment of patients or the completion of other clinical trial milestones.
Expenses related to clinical trials are accrued based on estimates and/or representations from service providers regarding work performed, including actual level of patient enrollment, completion of patient studies and progress of the clinical trials. Other incidental costs related to patient enrollment or treatment are accrued when reasonably estimable. If amounts and obligations to pay under clinical trial agreements are modified (for instance, as a result of changes in the clinical trial protocol or scope of work to be performed), the accruals are adjusted accordingly. Revisions to contractual payment obligations are charged to expense in the period in which the facts that give rise to the revision become reasonably certain.
General and Administrative
General and administrative expenses consist of salaries, benefits, and stock-based compensation for personnel in executive, finance and administrative functions, professional services and associated costs related to accounting, tax, audit, legal, intellectual property, consulting costs, conferences and travel, including allocated facility and general and administrative indirect overhead related costs to research and development expenses. General and administrative expenses are expensed in the period incurred.
Stock-Based Compensation
The Company estimates the fair value of stock options using the Black-Scholes option pricing model, which incorporates various assumptions including volatility, expected term and risk-free interest rate. Compensation related to service-based awards is recognized starting on the grant date on a straight-line basis over the vesting period, which is generally four years.
| F-9 |
The determination of the fair value of each stock award using this option-pricing model is affected by the Company’s assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, the fair value of the common stock at the date of grant, the expected term of the awards, the expected stock price volatility over the expected term of the awards, the risk-free interest rate, and the dividend rate as follows:
Fair Value of Common Stock—Prior to the IPO, given the absence of a public trading market, the Company’s Board of Directors considered numerous objective and subjective factors to determine the fair value of the Company’s common stock at each grant date. These factors included, but were not limited to: (i) contemporaneous third-party valuations of common stock; (ii) the prices for preferred stock sold to outside investors; (iii) the rights and preferences of preferred stock relative to common stock; (iv) the lack of marketability of the Company’s common stock; (v) developments in the business; and (vi) the likelihood of achieving a liquidity event, such as an IPO or sale of the business, given prevailing market conditions. The methodology to determine the fair value of the Company’s common stock included estimating the fair value of the enterprise using the “backsolve” method, which is a market approach that assigns an implied enterprise value by accounting for all share class rights and preferences based on the latest round of financing. The total equity value implied was then applied in the context of an option pricing model to determine the value of each class of the Company’s shares.
For grants issued post-IPO the closing price of the Company’s common stock as reported on the date of grant will determine the fair value of the Company’s common stock, as shares of the Company’s common stock are traded in the public market.
Expected Term—The expected term represents the period that the stock-based awards are expected to be outstanding. The Company determines the expected term using the simplified method for pre-IPO and post-IPO awards. The simplified method deems the term to be the average of the time-to-vesting and the contractual life of the options.
Expected Volatility—Given the absence of a public trading market, pre-IPO and post IPO, the expected volatility is estimated by taking the average historic price volatility for industry peers, consisting of several public companies in the Company’s industry that are either similar in size, stage, or financial leverage, over a period equivalent to the expected term of the awards.
Risk-Free Interest Rate—The risk-free interest rate is calculated using the average of the published interest rates of U.S. Treasury zero-coupon issues with maturities that are commensurate with the expected term.
Dividend Rate—The dividend yield assumption is zero as the Company has no plans to make dividend payments.
The Company generally granted stock options, pre-IPO, to its employees and consultants for a fixed number of shares with an exercise price equal to the fair value of the underlying shares at date of grant. For all post-IPO grants issued, the fair value will be the closing price of the Company’s common stock on the date of the grant. The Company accounts for all stock option grants using the fair value method and stock-based compensation is recognized as the underlying options vest.
Income Taxes
Deferred tax assets and liabilities are measured based on differences between the financial reporting and tax basis of assets and liabilities using enacted rates and laws that are expected to be in effect when the differences are expected to reverse. The Company records a valuation allowance for the full amount of deferred assets, which would otherwise be recorded for tax benefits relating to operating loss and tax credit carryforwards for all periods presented, as realization of such deferred tax assets cannot be determined to be more likely than not. Due to losses incurred for all periods presented, the Company does not record a tax provision or benefit for income taxes.
Emerging Growth Company and Smaller Reporting Company Status
The Company is an emerging growth company (“EGC”) as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) and may take advantage of reduced reporting requirements that are otherwise applicable to public companies. Section 107 of the JOBS Act exempts emerging growth companies from complying with new or revised financial accounting standards until private companies are required to comply with those standards. The Company has elected to use the extended transition period for complying with new or revised accounting standards.
We are also a “smaller reporting company,” as defined in Rule 12b-2 of the Exchange Act. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, like emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
| F-10 |
From time to time, new accounting pronouncements are issued by the FASB or other standard setting bodies and adopted by the Company as of the specified effective date. Unless otherwise discussed, the impact of recently issued standards that are not yet effective will not have a material impact on the Company’s financial position or results of operations upon adoption.
Recent Accounting Pronouncement
Recently Adopted Accounting Pronouncements
In February 2016, the FASB issued Accounting Standards Updates (“ASU”) 2016-02, Leases (Topic 842). The guidance requires lessees to recognize assets and liabilities related to long-term leases on the balance sheet and expands disclosure requirements regarding leasing arrangements. In July 2018, the FASB issued additional guidance, which offers a transition option to entities adopting the new lease standards, and a package of practical expedients an entity can elect to utilize to reduce the level of effort required for adoption. Under the transition option, entities can elect to apply the new guidance using a modified retrospective approach at the beginning of the year in which the new lease standard is adopted, rather than to the earliest comparative period presented in their financial statements. In November 2019, the FASB issued ASU 2019-10, Leases (Topic 842) (ASU 2019-10), deferring the effective date for the Company for fiscal years beginning after December 15, 2020, and interim periods within fiscal years beginning after December 15, 2021. In June 2020, the FASB issued ASU 2020-05, Leases (Topic 842) (ASU 2020-05), which further defers the effective date for the Company for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2021. Early adoption is permitted. The Company adopted ASU 2016-02 on January 1, 2022 and the adoption had no material impact to the Company’s financial statements.
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (ASU 2019-12), which simplifies the accounting for income taxes, and is effective on a prospective basis for annual reporting periods beginning after December 15, 2021, and for interim periods within fiscal years beginning after December 15, 2022, with early adoption permitted. The Company adopted ASU 2019-12 on January 1, 2022 and the adoption had no significant impact to the Company’s financial statements.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments—Credit Losses (Topic 326). The guidance represents a significant change in the accounting for credit losses model by requiring immediate recognition of management’s estimates of current expected credit losses (CECL). Under the prior model, losses were recognized only as they were incurred. The Company has determined that it has met the criteria of a smaller reporting company (“SRC”) as of November 15, 2019. As such, ASU 2019-10, Financial Instruments —Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842) —Effective Dates amended the effective date for the Company to be for reporting periods beginning after December 15, 2022. The Company adopted this ASU 2016-13 on January 1, 2023 and the adoption had no significant impact to the Company’s financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40) (ASU 2020-06): Accounting for Convertible Instruments and Contracts in an Entity, which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The updated guidance is effective on a prospective basis for annual reporting periods beginning after December 15, 2023 and for interim periods within those periods. The Company has early adopted on January 1, 2022 and the pronouncement did not have any material impact on the Company’s financial position or results of operations.
| F-11 |
3. Short-Term Marketable Securities
The tables summarize the Company’s short-term marketable securities as of December 31, 2022. There were no short-term marketable securities as of December 31, 2023 (in thousands):
Amortized Cost Basis | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||
| U.S. Treasury bills | $ | $ | $ | $ | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
4. Accrued Expenses
The components of accrued expenses as of December 31, 2023 and 2022 are as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Clinical trial | $ | $ | ||||||
| Employee benefits | ||||||||
| Other | ||||||||
| Total accrued expenses | $ | $ | ||||||
5. Fair Value Measurements
As
of December 31, 2023, and 2022, the Company held $
The following tables sets summarize the Company’s financial assets and liabilities, measured at fair value on a recurring basis by level within the fair value hierarchy, as of December 31, 2023, and 2022, (in thousands):
| Fair Value Measurements at December 31, 2023 using: | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Cash equivalents: | ||||||||||||||||
| Money market funds | $ | $ | $ | $ | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Liabilities: | ||||||||||||||||
| Common stock warrant liability | $ | $ | $ | $ | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
| Fair Value Measurements at December 31, 2022 using: | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Cash equivalents: | ||||||||||||||||
| Money market funds | $ | $ | $ | $ | ||||||||||||
| Available-for-sale securities: | ||||||||||||||||
| U.S. Treasury bills | ||||||||||||||||
| $ | $ | $ | $ | |||||||||||||
There were no transfers between Level 1, Level 2 or Level 3 during the periods presented. The Company had no other financial assets or liabilities that were required to be measured at fair value on a recurring basis.
| F-12 |
6. Commitments and Contingencies
Legal Proceedings
From time to time, the Company may become involved in legal proceedings arising in the ordinary course of business.
The Company was not subject to any material legal proceedings during the year ended December 31, 2023 and no material legal proceedings are subsequently outstanding or pending.
Guarantees and Indemnification
In the ordinary course of business, the Company enters into agreements that may include indemnification provisions. As permitted under Delaware law and in accordance with its bylaws, the Company indemnifies its officers and directors for certain events or occurrences while the officer or director is or was serving in such capacity. The Company is also party to indemnification agreements with its officers and directors. In some cases, the indemnification will continue after the termination of the agreement. The maximum potential amount of future payments that the Company could be required to make under these provisions is not determinable. The Company has never incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. The Company is not currently aware of any indemnification claims. Accordingly, the Company has not recorded any liabilities for these indemnification rights and agreements as of December 31, 2023.
Operating Leases
2021 Omnibus Equity Incentive Plan
On July 19, 2021, the Company’s Board of Directors adopted the RenovoRx, Inc. 2021 Omnibus Equity Incentive Plan (the “2021 Plan”). The 2021 Plan, which became effective immediately prior to the closing of the IPO, initially reserved shares of common stock, which included shares of common shares reserved but unissued under the Amended and Restated 2013 Equity Incentive Plan (the “2013 Plan”). The Company’s 2013 Plan was terminated immediately prior to the closing of the IPO; however, shares subject to awards granted under the 2013 Plan will continue to be governed by the 2013 Plan. In accordance with the terms of the 2021 Plan, on January 1, 2024, the number of shares reserved and available for issuance increased by shares.
Number of Stock Options | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Life | Aggregate Intrinsic Value | |||||||||||||
| Outstanding as of December 31, 2022 | $ | $ | ||||||||||||||
| Granted | $ | - | $ | - | ||||||||||||
| Exercised | ( | ) | $ | - | $ | - | ||||||||||
| Forfeited | ( | ) | $ | - | $ | - | ||||||||||
| Expired | ( | ) | $ | - | $ | - | ||||||||||
| Outstanding as of December 31, 2023 | $ | $ | ||||||||||||||
| Exercisable as of December 31, 2023 | $ | $ | ||||||||||||||
| Vested and expected to vest as of December 31, 2023 | $ | $ | ||||||||||||||
As of December 31, 2023, there was $ million of unrecognized stock-based compensation expense related to options granted but not yet amortized, which will be recognized over a weighted-average period of approximately years.
| F-13 |
| Options Outstanding | Options Exercisable | |||||||||||||||
| Range of Exercise Prices | Number of Shares | Weighted Average Remaining Contractual Life | Number of Shares | Weighted Average Remaining Contractual Life | ||||||||||||
| $ - $ | ||||||||||||||||
| $ - $ | ||||||||||||||||
| $ - $ | ||||||||||||||||
| $ - $ | ||||||||||||||||
| $ - $ | ||||||||||||||||
| $ - $ | ||||||||||||||||
| $ - $ | ||||||||||||||||
| Total | ||||||||||||||||
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Expected volatility | % – | % | % – | % | ||||
| Expected term (years) | – | – | ||||||
| Risk-free interest rate | % – | % | % – | % | ||||
| Dividend rate | % | % | ||||||
Changes on Level 3 Liabilities Measured at Fair Value on a Recurring Basis
The following table reflects the change in the Company’s Level 3 common warrant liability for the year ended December 31, 2023 (in thousands):
| Fair value as of December 31, 2022 | $ | |||
| Common warrants issued in connection with private placement | ||||
| Change in fair value | ( | ) | ||
| Fair value as of December 31, 2023 | $ |
The Company remeasures the fair value of its common warrant liability at each reporting date. It calculates the estimated fair value of the common warrant liability using a Monte Carlo simulation. The following table details the assumptions used in the Monte Carlo simulation to estimate the fair value of the common warrant liability as of December 31, 2023 and April 3, 2023 (the inception date), respectively:
| December 31, 2023 | April 3, 2023 | |||||||
| Stock price | $ | $ | ||||||
| Expected volatility | % | % | ||||||
| Expected term (years) | ||||||||
| Risk-free interest rate | % | % | ||||||
| Dividend rate | % | % | ||||||
During
the years ended December 31, 2023, and 2022, the Company recognized $
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| Total stock-based compensation expense | $ | $ | ||||||
In
January 2022, the Company granted shares of restricted common stock under the 2021 Plan to a consultant as partial consideration
for services rendered, with a deemed fair value of $ per share or $
| F-14 |
Common Stock Warrants
In
connection with the IPO, the Company issued warrants to purchase
The following is a summary of the common stock warrant activity during the year ended December 31, 2023:
| Shares Issuable Upon Exercise of Outstanding Warrants | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Life (in years) | Aggregate Intrinsic Value (in thousands) | |||||||||||||
| Outstanding as of December 31, 2022 | $ | $ | ||||||||||||||
| Issued | ||||||||||||||||
| Exercised | ( | ) | - | ( | ) | |||||||||||
| Expired | - | - | ||||||||||||||
| Outstanding as of December 31, 2023 | $ | $ | ||||||||||||||
8. Income Taxes
For the years ended December 31, 2023 and 2022, the Company’s income tax provision is zero due to a full valuation allowance against the deferred tax assets.
The differences between the tax provision (benefit) at the statutory federal tax rate and the tax provision (benefit) are as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Statutory federal income tax | $ | ( | ) | $ | ( | ) | ||
| Increase (decrease) resulting from: | ||||||||
| Change in valuation allowance | ||||||||
| Permanent items | ( | ) | ||||||
| Prior year true ups | ( | ) | ( | ) | ||||
| Tax credits | ( | ) | ( | ) | ||||
| State | ( | ) | ( | ) | ||||
| Other | ||||||||
| Income tax provision | $ | $ | ||||||
| F-15 |
The components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | $ | ||||||
| Tax credits carryforwards | ||||||||
| Stock-based compensation | ||||||||
| Fixed assets/intangible assets | ||||||||
| Charitable contributions | ||||||||
| Capitalized research | ||||||||
| Accruals and other | ||||||||
| Gross deferred tax assets | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Deferred tax assets | ||||||||
| Deferred tax liabilities: | ||||||||
| Unrealized gain | ||||||||
| Deferred tax liabilities | ||||||||
| Net deferred tax asset | $ | $ | ||||||
At
December 31, 2023, the Company had federal and state net operating loss (“NOL”) carryforward amounts of $
As
of December 31, 2023, the Company had federal and state tax credit carryforwards of $
The
Company follows Financial Accounting Standards Board No. 48, Accounting for Uncertainty in Income Taxes – an interpretation
of FASB No. 109, as codified in FASB ASC 740-10, Income Taxes. At December 31, 2023, unrecognized tax benefits related to
federal and state tax credits was $
Uncertain Income Tax Positions
The
total amount of unrecognized tax benefits as of December 31, 2023 is $
The following summarizes the activity related to the Company’s unrecognized tax benefits for the years ended December 31, 2022 and December 31, 2023 (in thousands):
| Balance at December 31, 2021 | $ | |||
| Tax positions related to the current year: | ||||
| Additions | ||||
| Balance at December 31, 2022 | ||||
| Tax positions related to the current year: | ||||
| Additions | ||||
| Balance at December 31, 2023 | $ |
| F-16 |
The Company’s policy is to account for interest and penalties as income tax expense. As of December 31, 2023, the Company had no interest related to unrecognized tax benefits. No amounts of penalties related to unrecognized tax benefits were recognized in the provision for income taxes. We do not anticipate any significant change within twelve months of this reporting date.
The Company files income tax returns in the U.S. federal jurisdiction and various state jurisdictions. The Company is subject to U.S. federal and state income tax examination for calendar tax years beginning in 2011 due to net operating losses that are being carried forward for tax purposes.
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Numerator: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Denominator: | ||||||||
| Weighted average shares used in computing net loss per share – basic and diluted | ||||||||
| Net loss per share – basic and diluted | $ | ) | $ | ) | ||||
For the years ended December 31, 2023 and 2022, the Company had a net loss and as such, all outstanding shares of potentially dilutive securities were excluded from the calculation of diluted net loss per share as the inclusion would be anti-dilutive.
| Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Options to purchase common stock | ||||||||
| Total | ||||||||
10. Related Party Transactions
In
January 2018, the Company entered into a consulting agreement with one of the Company’s co-founders, Dr. Ramtin Agah, pursuant
to which Dr. Agah provides consulting services as the Company’s Chief Medical Officer by overseeing Company-sponsored clinical
trials. The agreement, which was amended on September 1, 2019, and November 11, 2021, respectively, continues in force for as long as
Dr. Agah is providing consulting services and may be terminated by either party on 30 days’ notice. Dr. Agah was awarded (i) options
to purchase shares of the Company’s common stock in May 2017, which have vested, (ii) options to purchase shares
of the Company’s common stock in July 2018, which have vested, (iii) options to purchase shares of the Company’s common
stock in June 2021, which vest ratably over 24 months from the vesting commencement date of May 14, 2021, (iv) options to purchase of
shares of the Company’s common stock in September 2021, which vest ratably over 48 months from the vesting commencement
date of August 26, 2021, and (v) options to purchase shares of the Company’s common stock in March 2022, which vest ratably
over 48 months from the vesting commencement date of August 26, 2021. In December 2018, Dr. Agah’s agreement was amended to provide
that he would receive cash compensation of $
| F-17 |
11. Employee Benefit Plans
In
January 2022, the Company established a defined contribution plan under Section 401(k) of the Internal Revenue Code (the “401(k)
plan”). The 401(k) plan covers all employees who meet defined minimum age and service requirements. Employee contributions are
voluntary and are determined on an individual basis, limited to the maximum amount allowable under U.S. federal tax regulations. The
Company makes matching contributions of up to 4% of the eligible employees’ compensation to the 401(k) plan. During the year ended
December 31, 2023, the Company made contributions to the 401(k) plan of $
12. Subsequent Events
On
January 26, 2024, the Company entered into a series of subscription agreements, in connection with a private placement offering to
On February 23, 2024, the Company received a written notice from Nasdaq that the Company had, as of the date of the Notice, failed to meet the Minimum Stockholders’ Equity Requirement and that Nasdaq would commence delisting proceedings against the Company unless the Company timely requests a hearing before the Nasdaq Hearing Panel. On February 28, 2024, the Company provide written notice to request a hearing, which request will stay any delisting or suspension action by the Nasdaq staff at least pending the issuance of the Hearing’s Panel decision and the expiration of any extension that may be granted to the Company following the hearing. The hearing will be held on April 23, 2024.
| F-18 |