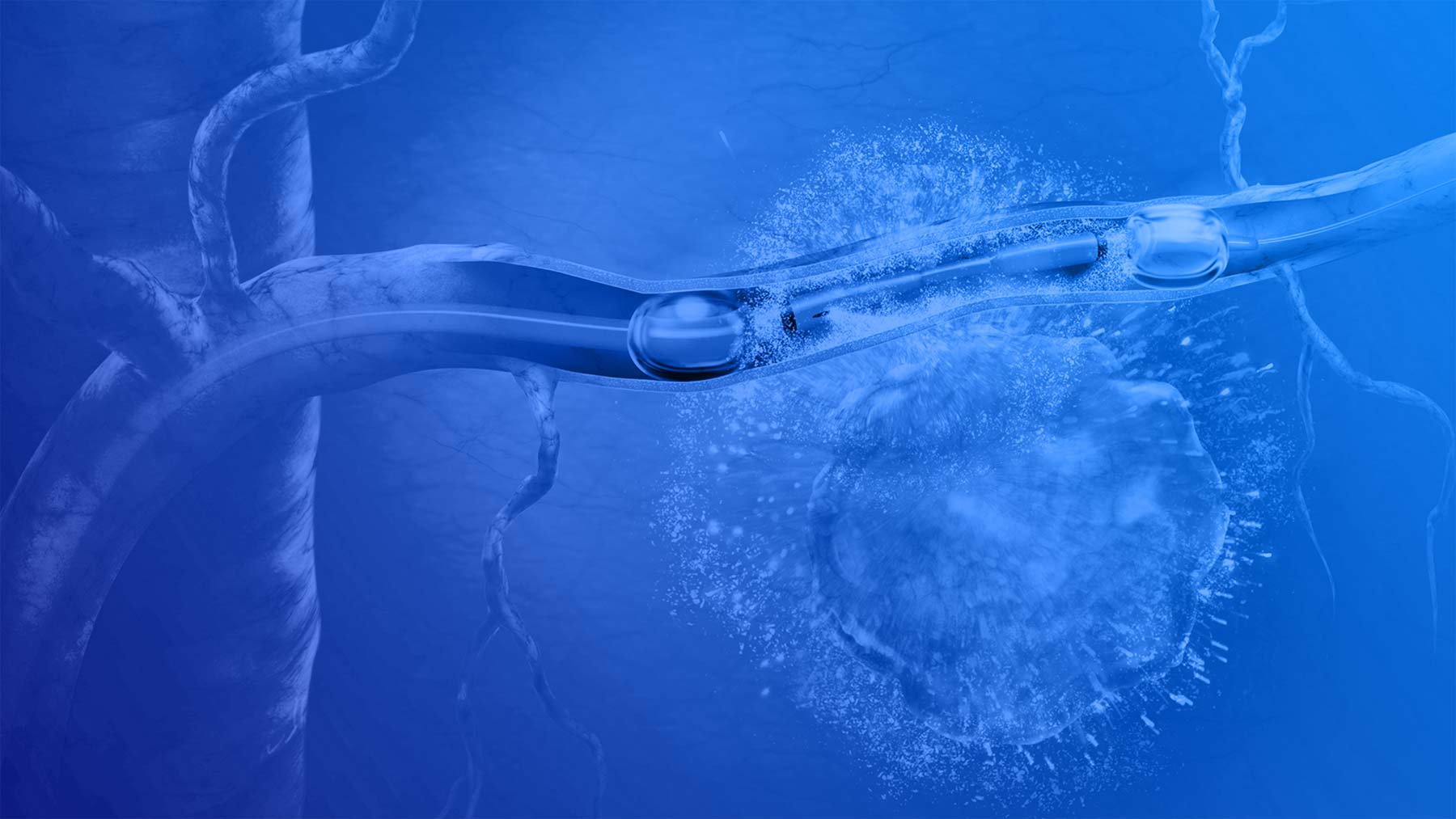
*RenovoCath is currently cleared for the indication below. The Company is evaluating its novel combination oncology product candidate (intra-arterial gemcitabine, known as IAG) in the ongoing Phase III TIGeR-PaC trial. IAG is being evaluated by the Center for Drug Evaluation and Research (the drug division of FDA) under a U.S. investigational new drug application that is regulated by the FDA’s 21 CFR 312 pathway. Based on completed studies, intra-arterial delivery of chemotherapy via Trans-Arterial Micro-Perfusion (TAMP™), which targets delivery of treatment in proximity to the tumor tissue within the pancreas using the vasa vasorum pathway, reduced tolerability issues associated with systemic chemotherapy and presented early signals of improved patient survival. The study, called TIGeR-PaC (“Intra-Arterial Gemcitabine vs. IV Gemcitabine and Nab-Paclitaxel Following Radiotherapy for LAPC”), is evaluating the combination oncology product candidate (IAG) which is not yet cleared or approved by FDA for this use and not yet available for commercial sale.
Indication for Use: RenovoCath is intended for the isolation of blood flow and delivery of fluids, including diagnostic and/or therapeutic agents, to selected sites in the peripheral vascular system. RenovoCath is also indicated for temporary vessel occlusion in applications including arteriography, preoperative occlusion, and chemotherapeutic drug infusion. RenovoCath is intended for general intravascular use in the peripheral vasculature in arteries 3mm and larger. The RenovoCath is intended for use in arteries from 3mm in diameter for vessel entry and to occlude vessels ranging between 3mm to 11mm in diameter. The diagnostic and/or therapeutic agents are to be used in accordance with specifications outlined by the respective agent manufacturer. For detailed information on RenovoCath including warnings, precautions, and contraindications, please refer to the product IFU on the RenovoRx website: https://renovorx.com/wp-content/uploads/2025/04/IFU-10004-Rev.-G-Universal-IFU.pdf.
 Vince Scully, 74, was diagnosed with Stage III pancreatic cancer in June, and initially underwent the standard treatment of chemotherapy and radiation. His cancer was inoperable but had not metastasized to other organs.
Vince Scully, 74, was diagnosed with Stage III pancreatic cancer in June, and initially underwent the standard treatment of chemotherapy and radiation. His cancer was inoperable but had not metastasized to other organs.